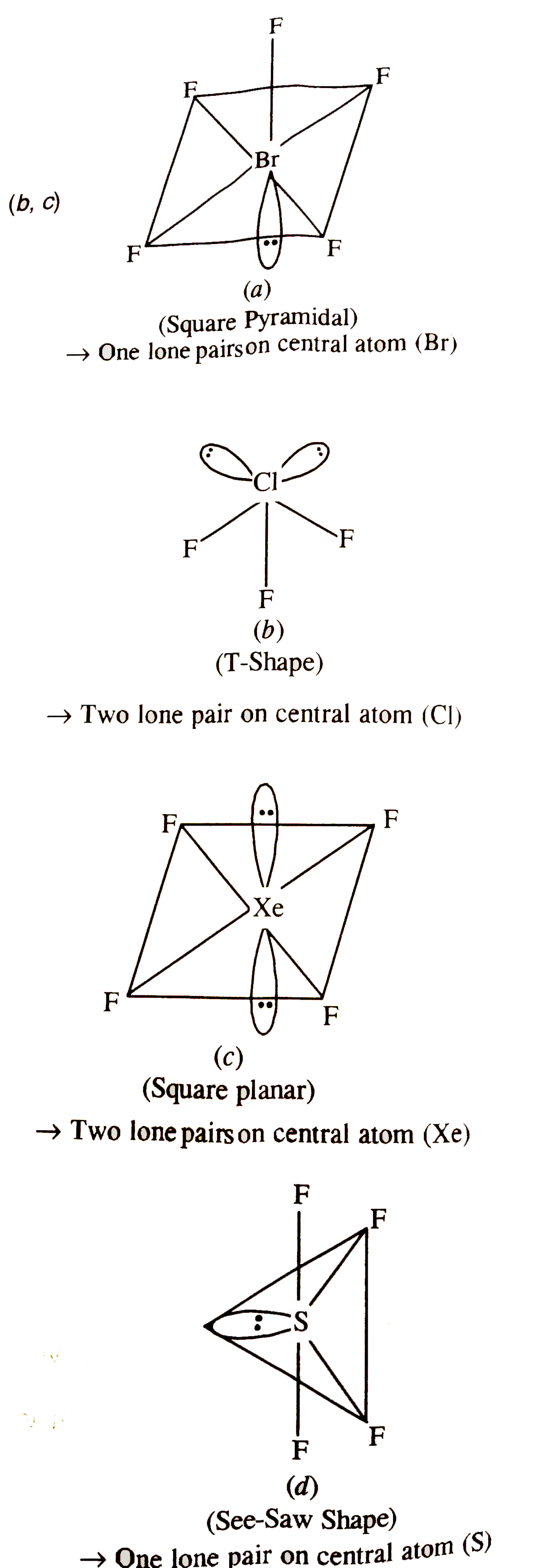A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise REASON TYPE QUESTIONS|16 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise MATRIX-MATCH TYPE QUEDTIONS|6 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise STRAIGHT OBJECTIVE TYPE MCQs (SINGLE CORRECT OPTION)|1 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
DINESH PUBLICATION|Exercise Unit test|20 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
DINESH PUBLICATION|Exercise All Questions|310 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE -MULTIPLE CHOICE ANSWER TYPE MCQs
- CO(2) is iso-structural with :
Text Solution
|
- Which of the following have identicla bond order ?
Text Solution
|
- Iso-structural species among the folwong are (l) CH(3)^(+)(ll)H(3)O^(+...
Text Solution
|
- The compound(s) with two lone pairs of electron on the central atom is...
Text Solution
|
- According to molecular arbital theory,
Text Solution
|