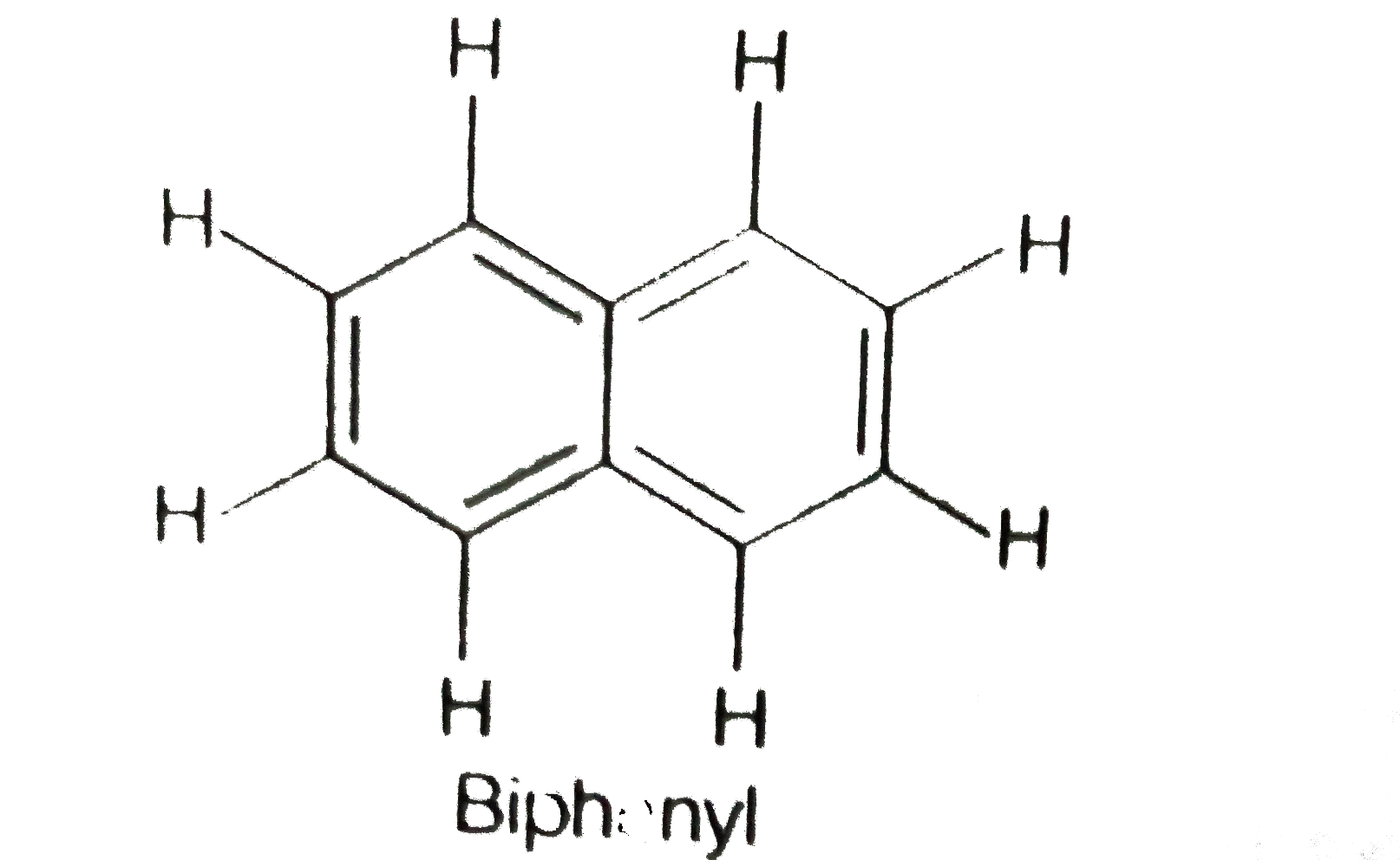
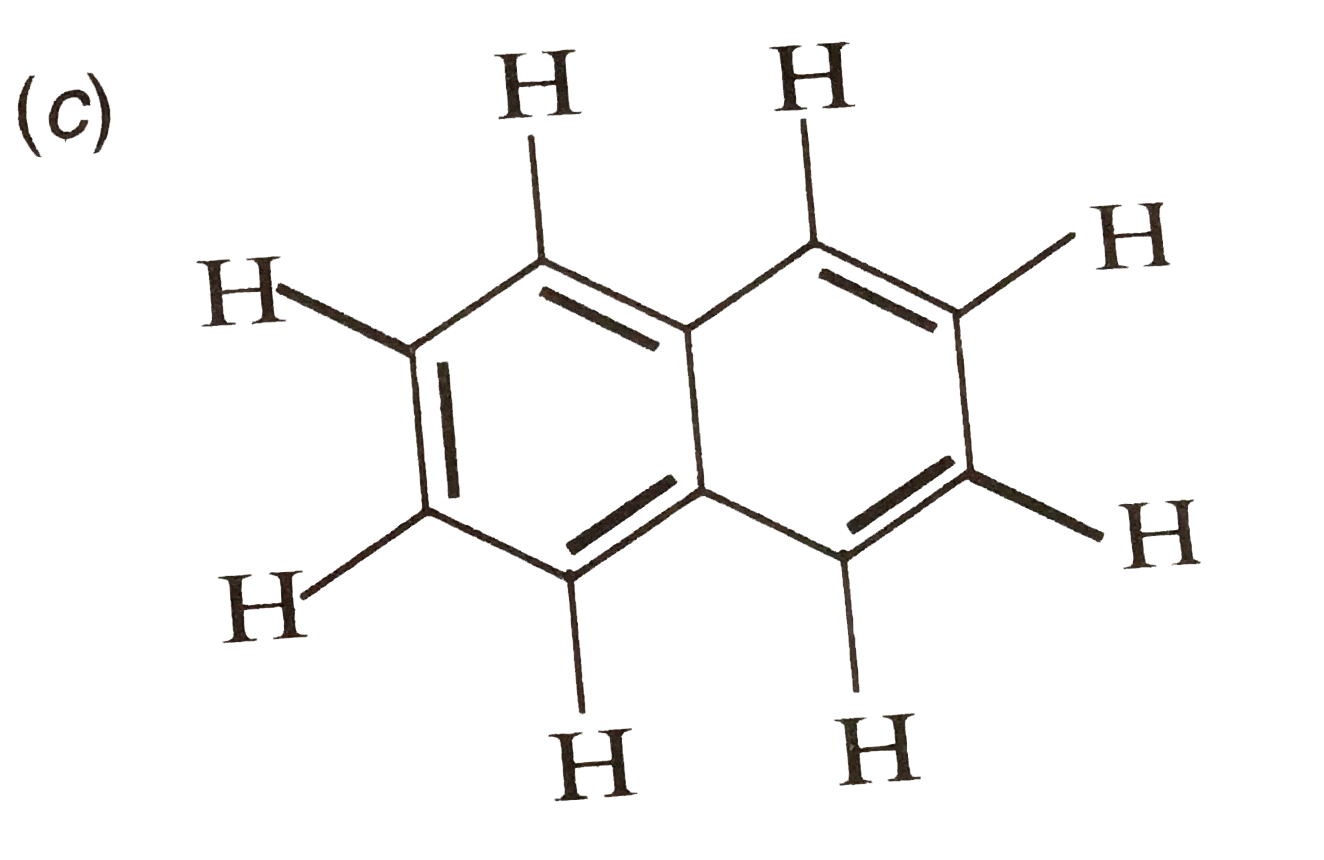
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise MULTIPLE CHOICE QUESTIONS (TYPE -II)|1 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise III.MATCHING TYPE QUESTIONS|5 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise MATRIX-MATCH TYPE QUEDTIONS|6 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
DINESH PUBLICATION|Exercise Unit test|20 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
DINESH PUBLICATION|Exercise All Questions|310 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE -MULTIPLE CHOICE QUESTIONS (TYPE -1)
- In NO(3)^(-) ion, the number of bond pair and lone pair of electrons o...
Text Solution
|
- Which of the following species does not have tetraahedral geometry ?
Text Solution
|
- Number of pi bonds and sigma bonds in the following structure is
Text Solution
|
- Which molecule/ion out of the following does not contain unpaired elec...
Text Solution
|
- In which of the following substances will hydrogen bond be strongest ?
Text Solution
|
- In which of the following substances will hydrogen bond be strongest?
Text Solution
|
- If the electronic configuration of an element is 1s^(2)2s^(2)2p^(2)3s^...
Text Solution
|
- Which of the folowing angles corresponds to sp^(2) hybridisation ?
Text Solution
|
- Stable from of A may be represented by the formula :
Text Solution
|
- Stable from of C may be represented by the formula :
Text Solution
|
- The molecular formula of the compound formed from B and C will be :
Text Solution
|
- The bond between B and C will be
Text Solution
|
- Which of the following orderof energies of molecular orbitals of N(2) ...
Text Solution
|
- Which of the following statement is not correct from the view point of...
Text Solution
|
- Which of the following options represents the correct bond order? Th...
Text Solution
|
- The electronic configuration of the outer most shell of the most elect...
Text Solution
|
- Amongst the following elements (whose electronic configuration an gi...
Text Solution
|
- Which of the following attain the linear structure ?
Text Solution
|
- CO is isoelectronic with
Text Solution
|
- Which of the following species have the same shape?
Text Solution
|