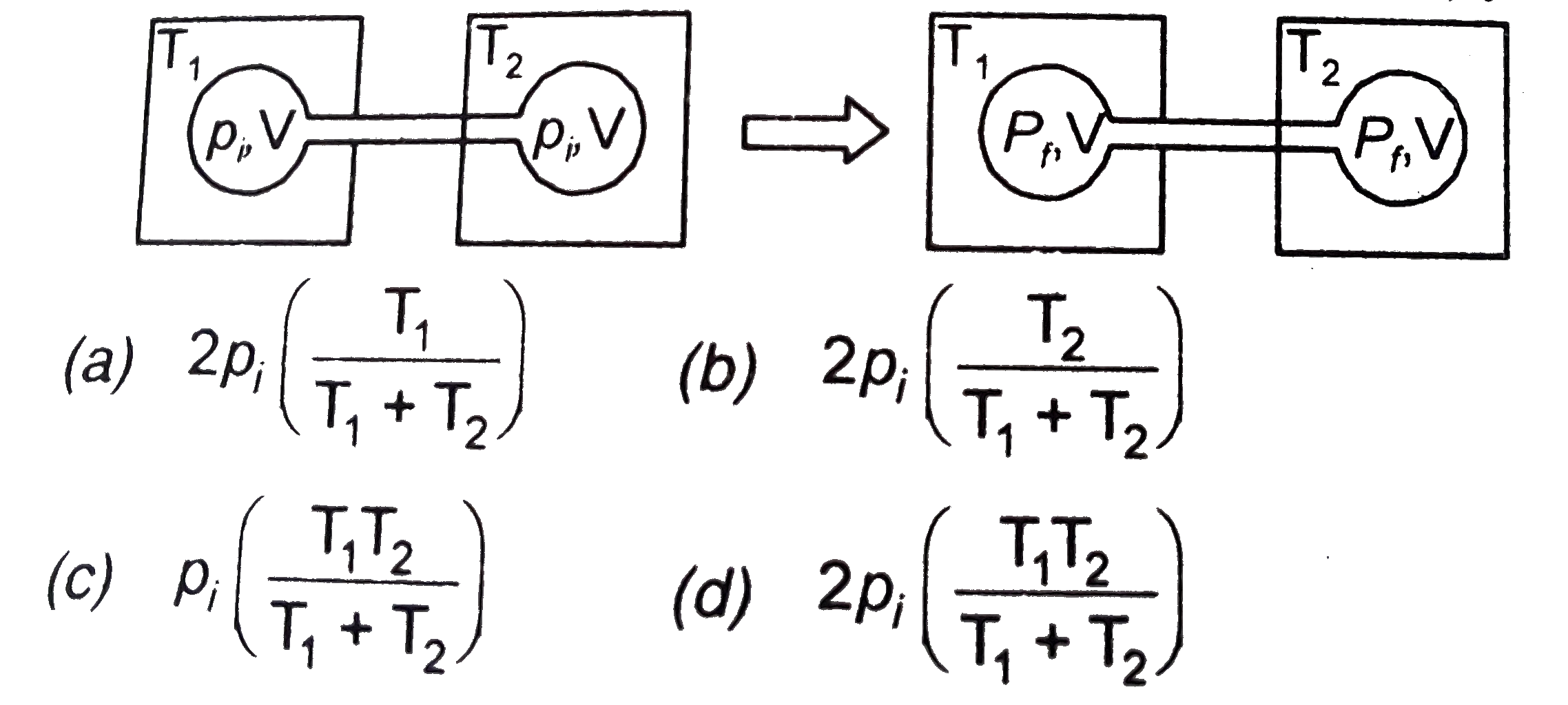A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER : GASES AND LIQUIDS
DINESH PUBLICATION|Exercise Comphension 1|5 VideosSTATES OF MATTER : GASES AND LIQUIDS
DINESH PUBLICATION|Exercise Comphension 2|5 VideosSTATES OF MATTER : GASES AND LIQUIDS
DINESH PUBLICATION|Exercise Question Bank (MCQ)|26 VideosSTATES OF MATTER (SOLID STATE CHEMISTRY)
DINESH PUBLICATION|Exercise ULTIMATE PREPARATORY PACKAGE|21 VideosSTRUCTURE OF ATOM
DINESH PUBLICATION|Exercise Reason Type Questions|1 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-STATES OF MATTER : GASES AND LIQUIDS-Select the correct answer
- The term which accounts for intermolecular force in van der Waal's equ...
Text Solution
|
- As the temperature is raised from 20^(@)C to 40^(@)C the averge kineti...
Text Solution
|
- In van der Waals' equation of state of the gas law the constnat 'b' is...
Text Solution
|
- The volume of a certain mass of gas at N.T.P. is 27.5 cm^(3). What pre...
Text Solution
|
- At 27^(@)C under one atmosphere pressure, a gas occupies a volume of V...
Text Solution
|
- Hydrogen gas occupies a volume of 18 litres at 27^(@)C and under a pr...
Text Solution
|
- what volume of hydrogen gas , at 273 K and 1 atm pressure will be con...
Text Solution
|
- Which one of the following statement is not true about the effect of a...
Text Solution
|
- Equal masses of methane and oxygen are mixed in an empty container at ...
Text Solution
|
- The density of a gas a is twice that of gas B. Molecular mass of A is ...
Text Solution
|
- At what temperature, the r.m.s. velocity of a gas measured at 50^(@)C ...
Text Solution
|
- The compressibility factor for a real gas at high pressure is .
Text Solution
|
- Mass of 112 cm^(3) of NH(3) gas at S.T.P. is
Text Solution
|
- For gaseous state, if most probable speed is denoted by C^(**) average...
Text Solution
|
- If Z is a compressibility factor, van der Waals' equation at low press...
Text Solution
|
- The statement that is NOT correct is :
Text Solution
|
- The intermolecular interaction that is dependent on the inverse cube o...
Text Solution
|
- The compressibility factor (Z) for one mole of a gas is more than one...
Text Solution
|
- Two closed bulb of euqal volume (V) containing an ideal gas initially ...
Text Solution
|
- Critical density of a gas having molecular mass 39 g mol^(-1) is 0.1 g...
Text Solution
|