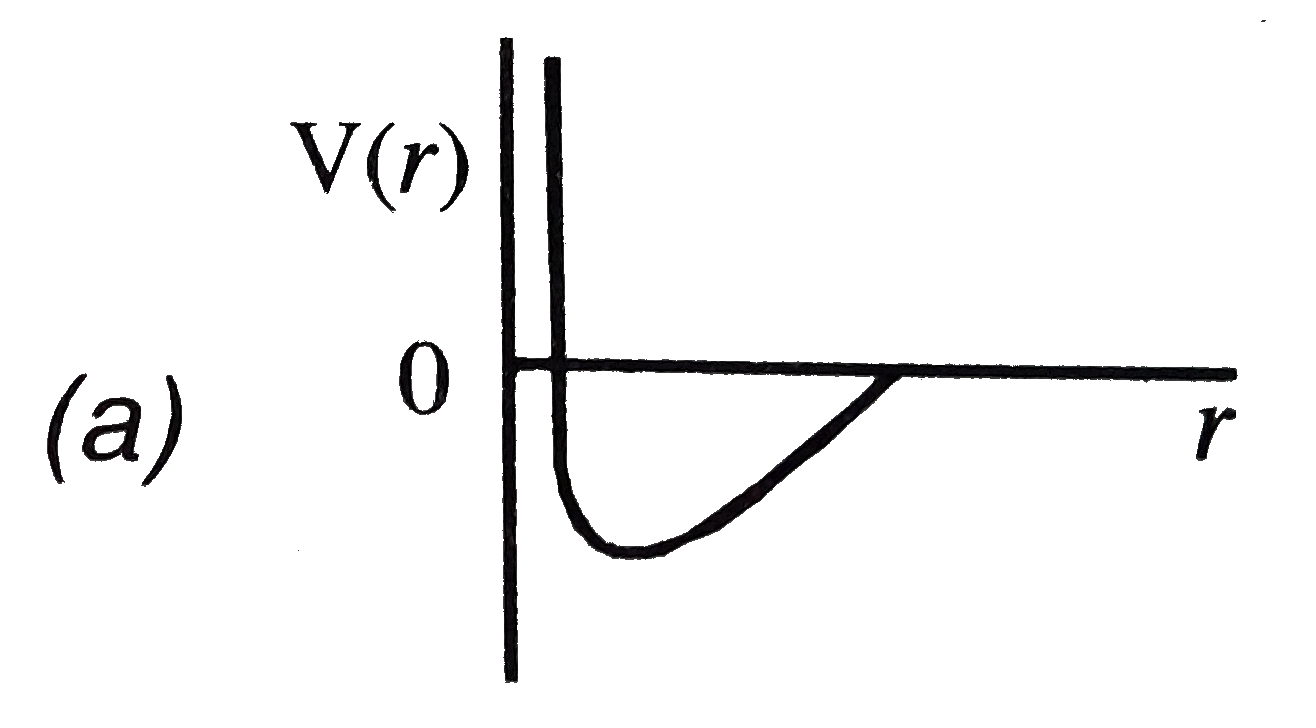
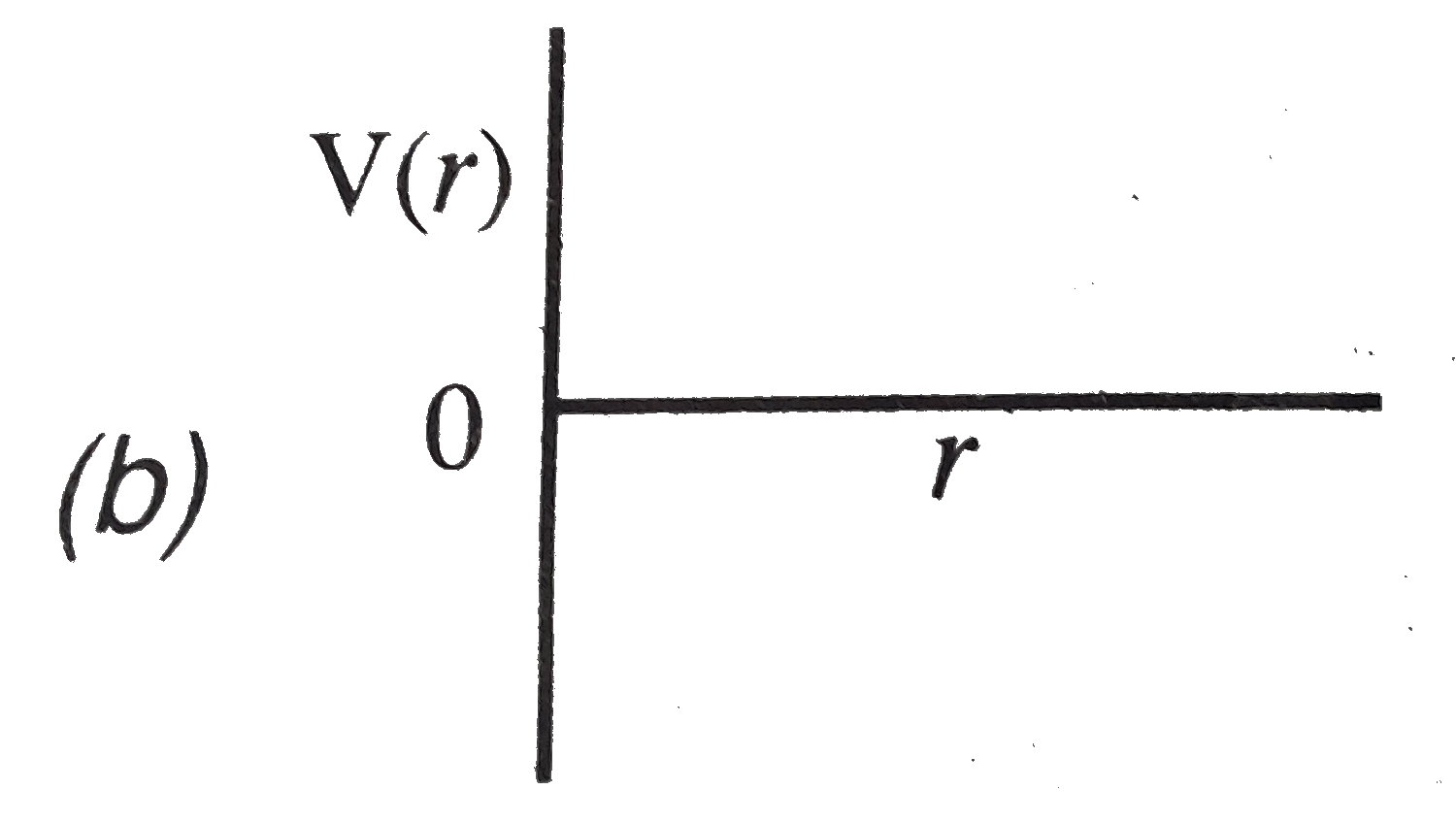
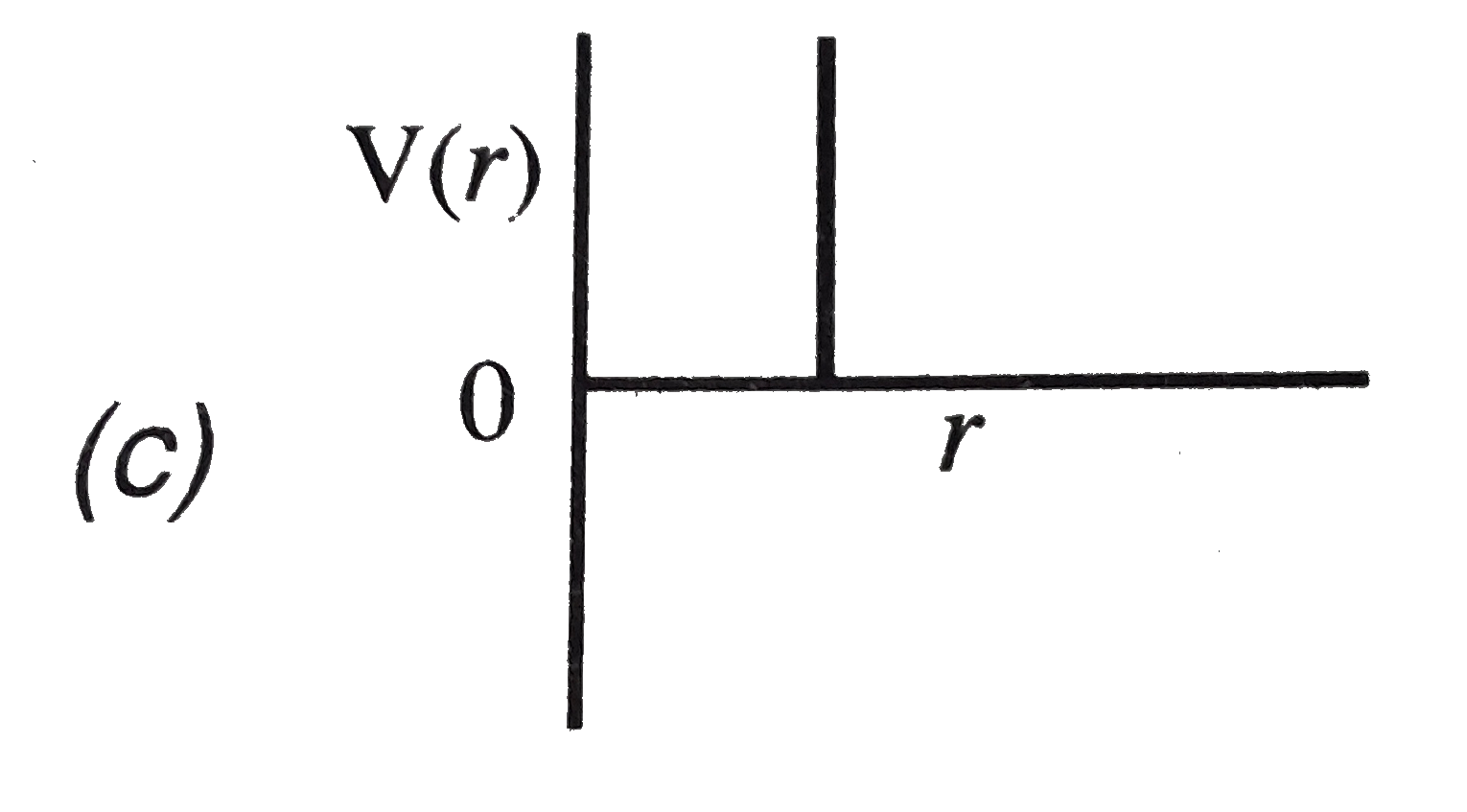
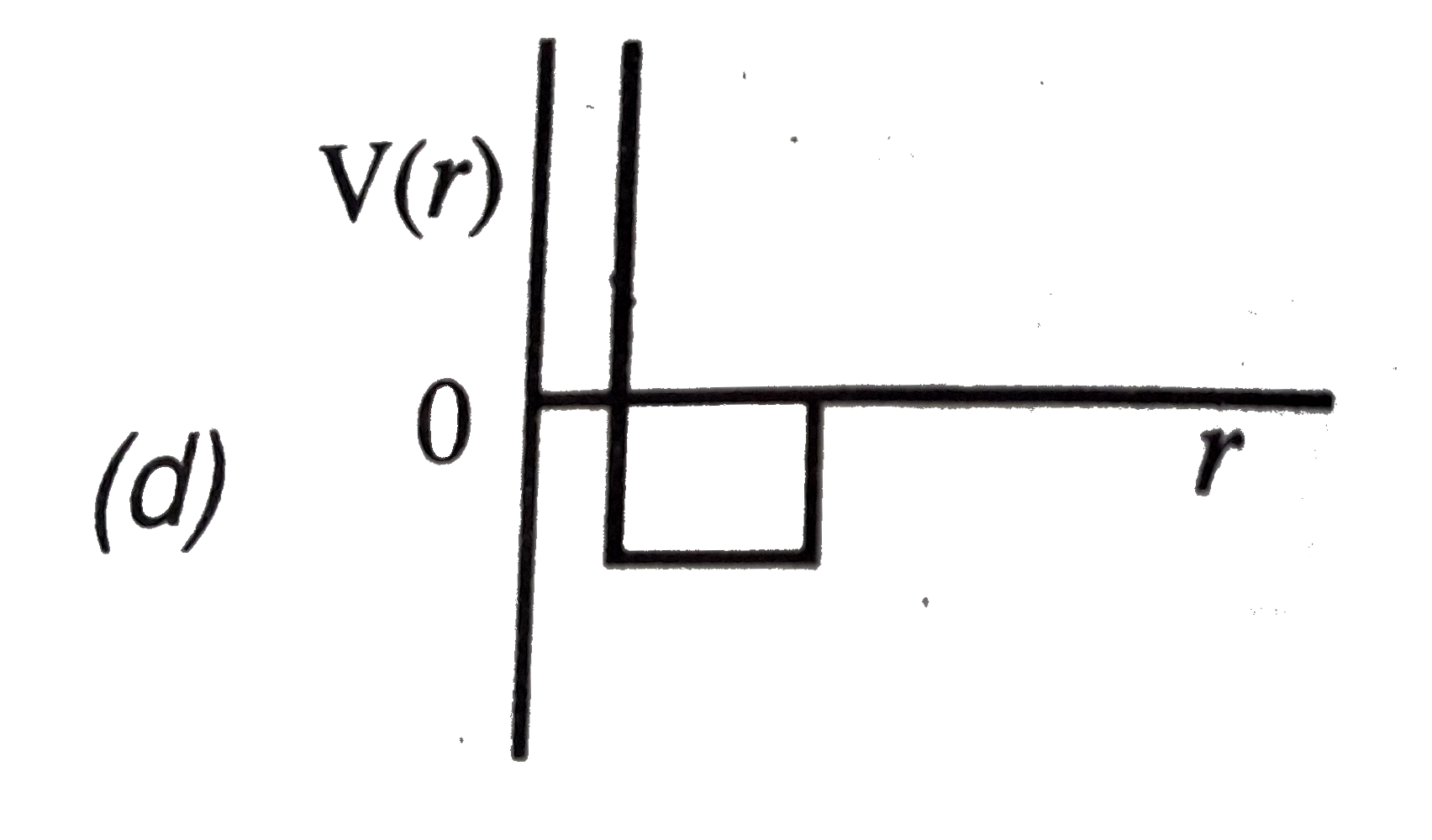
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER : GASES AND LIQUIDS
DINESH PUBLICATION|Exercise Assertion and Reason|11 VideosSTATES OF MATTER : GASES AND LIQUIDS
DINESH PUBLICATION|Exercise Statement Type Question|5 VideosSTATES OF MATTER : GASES AND LIQUIDS
DINESH PUBLICATION|Exercise Comphension 4|2 VideosSTATES OF MATTER (SOLID STATE CHEMISTRY)
DINESH PUBLICATION|Exercise ULTIMATE PREPARATORY PACKAGE|21 VideosSTRUCTURE OF ATOM
DINESH PUBLICATION|Exercise Reason Type Questions|1 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-STATES OF MATTER : GASES AND LIQUIDS-Straight Objective Type (MCQ)
- The compressibility of a gas is less than unity at N.T.P. Therefore.
Text Solution
|
- The r.m.s. velocity of hydrogen is sqrt(7) times the r.m.s. velocity 1...
Text Solution
|
- At 100^(@)C and 1 atm, if the density of the liquid water is 1.0 g cm^...
Text Solution
|
- The root mean square velocity of an ideal gas to constant pressure var...
Text Solution
|
- Which of the following volume-temperature (V-I) plots represents the b...
Text Solution
|
- When the temperature is increased surface tension of water .
Text Solution
|
- Positive deviation from ideal behaviour takes place because of
Text Solution
|
- The root mean square speed of one mole of a monoatomic gas having mole...
Text Solution
|
- The ratio of the rate of diffusion of helium and methane under identic...
Text Solution
|
- The given graph represents the variations of compressibility factor Z=...
Text Solution
|
- One mole of a monoatomic real gas satisfies the equation p(V-b)=RT wh...
Text Solution
|
- For one mole of a van der Waals gas when b =0 and T =30 K the PV vs1//...
Text Solution
|
- Which of the following gives straight line plot, keeping the third par...
Text Solution
|
- Which of the following are incorrect?
Text Solution
|
- Units of pressure are :
Text Solution
|
- Which of the following are not the units of gas constant (R) ?
Text Solution
|
- If a gas is allowe to expand at constant temperature,
Text Solution
|
- A gas described by van der Waals equation .
Text Solution
|
- The graph of P vs V is given at temperature and number of moles : ...
Text Solution
|
- Which of the following statements are correct ?
Text Solution
|