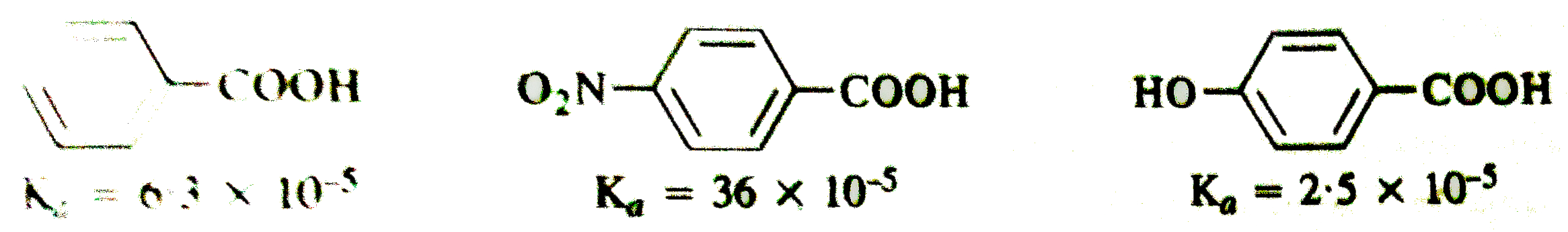Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC CHEMISTRY - SOME BASIC PRINCIPLES & TECHNIOUES
DINESH PUBLICATION|Exercise VALUE BASED QUESTION|3 VideosORGANIC CHEMISTRY - SOME BASIC PRINCIPLES & TECHNIOUES
DINESH PUBLICATION|Exercise Problems For Practice|39 VideosORGANIC CHEMISTRY - SOME BASIC PRINCIPLES & TECHNIOUES
DINESH PUBLICATION|Exercise SHORT ANSWER TYPE QUESTION|33 VideosNUCLEAR AND RADIO CHEMISTRY
DINESH PUBLICATION|Exercise Evaluate Yourself|45 VideosP BLOCK ELEMENTS (GROUP 13 AND 14 )
DINESH PUBLICATION|Exercise Straight obj.|17 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ORGANIC CHEMISTRY - SOME BASIC PRINCIPLES & TECHNIOUES-CONCEPT BASED QUESTION
- Predict the nature of the M-effect when 'Cl' and 'NO(2)' groups are at...
Text Solution
|
- Arrange the following in order of increasing acidic strength (i) HCO...
Text Solution
|
- Dissociation constant of benzoic acid, p-nitro benzoic acid and p-hydr...
Text Solution
|
- Which out of each pair is expected to be a stornger acid ? (a) CH(3)...
Text Solution
|
- Pick the electrophiles and nucleophiles from the following : (a) NH(...
Text Solution
|
- Which of the following statemets are false ? Assign suitable explanati...
Text Solution
|
- Suggest a method to separate the constituents from the following mixtu...
Text Solution
|
- How will you separate the constituents from a mixture of H(2)S and SO(...
Text Solution
|
- Point out the false statements from the following and rectify them : ...
Text Solution
|
- Select the principal functional group when the following groups are pr...
Text Solution
|
- Give the names of the following when these are not the part of the par...
Text Solution
|
- Give the names as secondary suffixes and primary suffixes for the foll...
Text Solution
|
- Designate each carbon atom as primary, secondary, tertiary and quanter...
Text Solution
|
- A certain substance contains only carbon and hydrogen and its molecula...
Text Solution
|
- Can we separate two liquids A (b.p. 353 K) and B (b.p. 365 K) present ...
Text Solution
|
- Under what condition can the process of steam distillation be used ?
Text Solution
|
- Lassaigne's extract is prepared in distilled water and not in tap wate...
Text Solution
|
- Why is impure glycerol by distillation under reduced pressure ?
Text Solution
|
- Hydrazine does not give Lassaigne's test for nitrogen. Why ?
Text Solution
|
- Can we use potassium in place of sodium for preparing Lassaigne's extr...
Text Solution
|