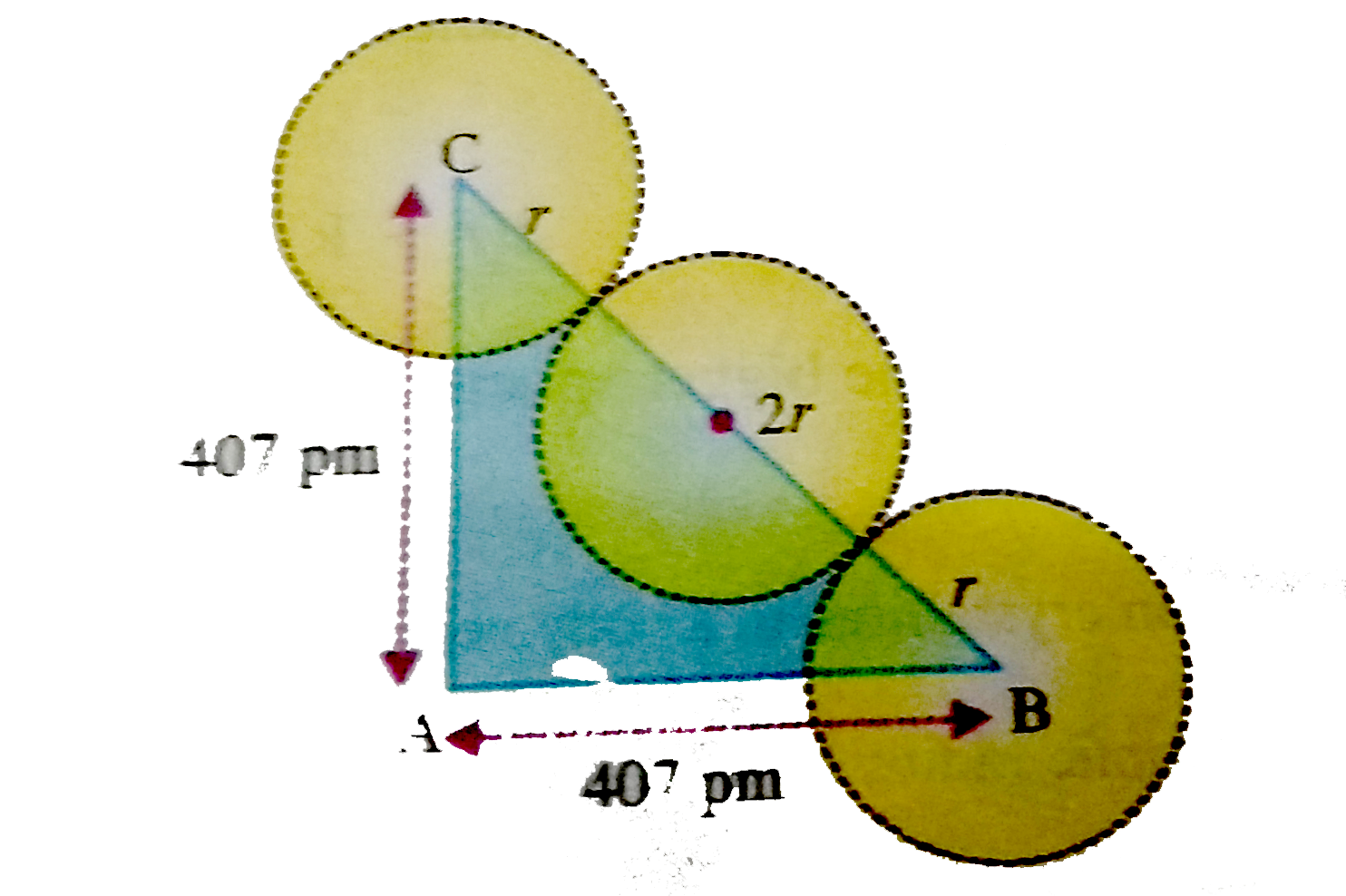
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-SOLID STATE-Brain storming
- Silver metal crystallises in a cubic closed- packed arrangement with t...
Text Solution
|
- Ice crystallises in hexagonal lattice having volume of unit cell is 13...
Text Solution
|
- At what angle for the first - order diffraction, spacing between two p...
Text Solution
|
- It is stated that ZnS does not crystallise in the NaCl structure it is...
Text Solution
|
- In solid ammonia, each NH(3) molecule has six other NH(3) molecules as...
Text Solution
|
- The density of KBr is 2.75 g cm^(-3). The length of the unit cell is 6...
Text Solution
|
- In a cubic packed structure of mixed oxides the lattice is made up of ...
Text Solution
|
- If there elements X, Y & Z crystallize in cubic solid latice with X at...
Text Solution
|
- The limiting radius ratios of the complexes [Ni(CN)(4)]^(2-) and [NiCl...
Text Solution
|
- KCl crystallises in the same type of lattice as does NaCl Given that r...
Text Solution
|
- If the unit cell of a mineral has cubic close packed (ccp) array of ox...
Text Solution
|