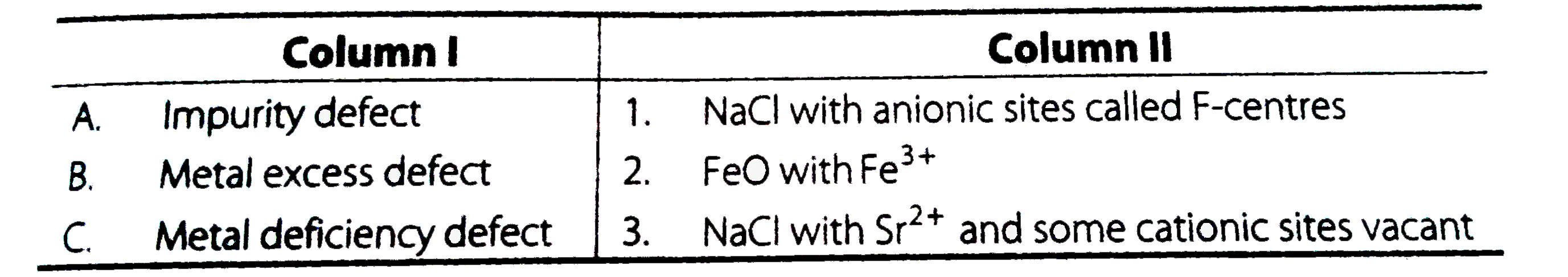Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLID STATE
DINESH PUBLICATION|Exercise ASSIGNMENT|134 VideosSOLID STATE
DINESH PUBLICATION|Exercise multiple choice|48 VideosSOLID STATE
DINESH PUBLICATION|Exercise PROBLEMS|71 VideosREDOX REACTIONS
DINESH PUBLICATION|Exercise Ultimate Preparation|9 VideosSOLUTIONS
DINESH PUBLICATION|Exercise ULTIMATE PREPARATORY PACKAGE|10 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-SOLID STATE-MULTIPLE CHOCIE QUESTION (TYPE-I)
- An excess of potassium ions makes KCL crystals appear violet or l...
Text Solution
|
- the number of tetrahedral voids per unit cell in NaCl crystal is ...
Text Solution
|
- Amorphous solids can also be callled ………….. .
Text Solution
|
- A perfect crystal of silicon (fig) is doped with some elements a...
Text Solution
|
- Which of the following statements are correct ?
Text Solution
|
- Which of the following features are not shown by quartz glass ?
Text Solution
|
- Which of the following cannot be regarded as molecular solid ?
Text Solution
|
- In which of the following arrangements, Octahedral voids are forme...
Text Solution
|
- Frenkel defect is also known as ……… .
Text Solution
|
- Which of the following defects decrease the density ?
Text Solution
|
- match the defects given in column I with the statements in given...
Text Solution
|
- match the type of unit cell given column I with the features iv...
Text Solution
|
- match the types of defect given in column I with the statement...
Text Solution
|
- match the items given in column I with the items given in colum...
Text Solution
|
- Match the type of packing given in column I with the iterms give...
Text Solution
|
- Assertion :- (a) the total number of atoms present in a simple c...
Text Solution
|
- Assertion :- (A) Graphite is good conductor of electricity however ...
Text Solution
|
- Assertion :- (A) total number of octahedral voids present in ...
Text Solution
|
- Assertion : The packing efficiency is maximum for the fcc structure. ...
Text Solution
|
- Assertion :-(A) semiconductors are solids with conductivites in t...
Text Solution
|