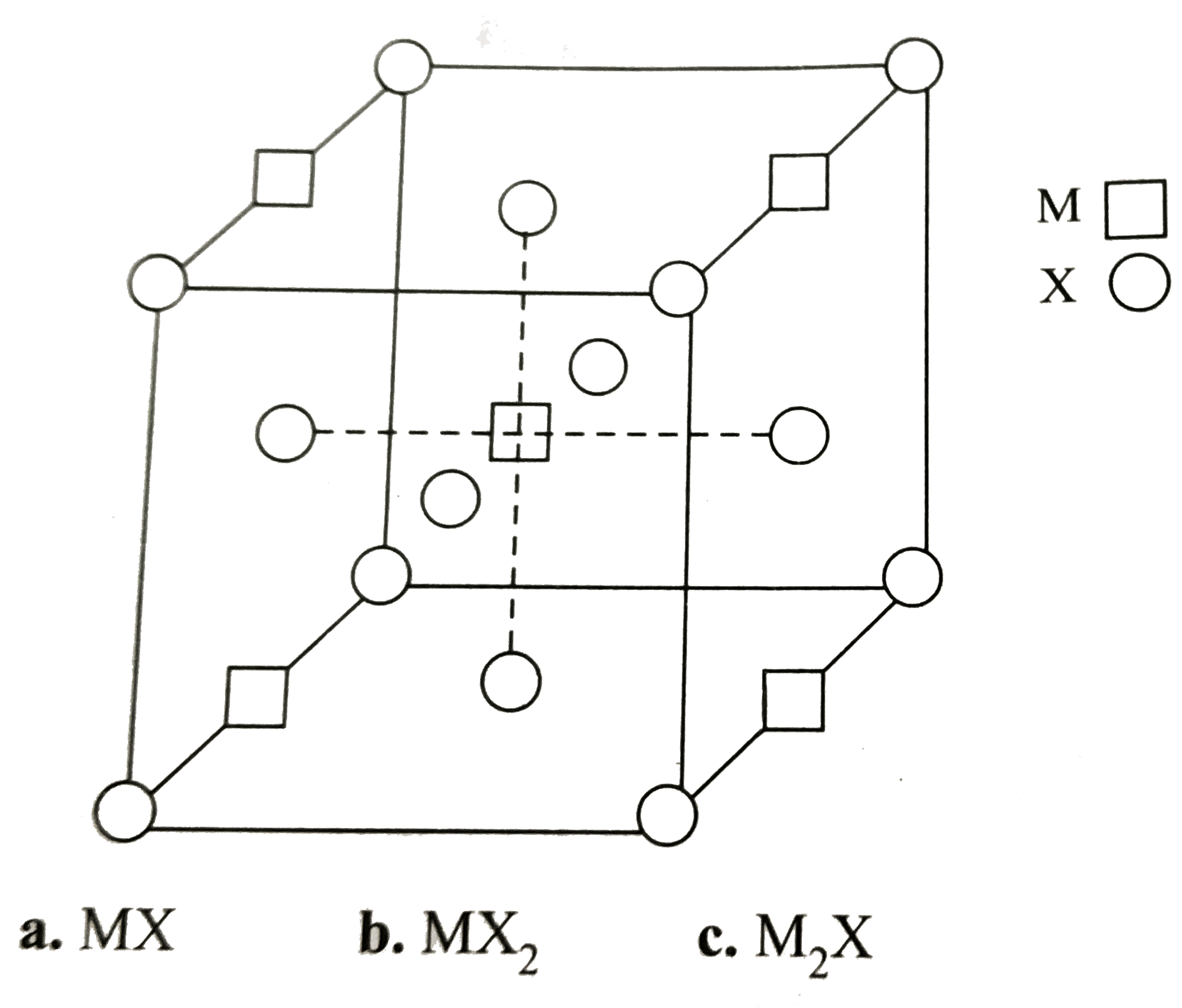
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLID STATE
DINESH PUBLICATION|Exercise A.R|27 VideosSOLID STATE
DINESH PUBLICATION|Exercise Integer|13 VideosSOLID STATE
DINESH PUBLICATION|Exercise comprehension|16 VideosREDOX REACTIONS
DINESH PUBLICATION|Exercise Ultimate Preparation|9 VideosSOLUTIONS
DINESH PUBLICATION|Exercise ULTIMATE PREPARATORY PACKAGE|10 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-SOLID STATE-Straight objective
- A substance A(x)B(y) crystallises in a face centred cubic (fcc) latti...
Text Solution
|
- The packing efficiency of the two dimensional square unit cell shown b...
Text Solution
|
- A compound M(p)X(q) has cubic close packing (ccp) arrangement of X. It...
Text Solution
|
- The arrangement of X^(ɵ) ions around A^(o+) ion in solid AX is given i...
Text Solution
|
- In the closest packing of atoms
Text Solution
|
- Incorrect option (s) about NaCl structure is/are
Text Solution
|
- CsCl structure is interchanged into NaCl structure. This can be done b...
Text Solution
|
- Which of the following statements are correct ?
Text Solution
|
- Glasses and plastics are
Text Solution
|
- Which is true ? If radius ratio r^(+)//r^(-)
Text Solution
|
- Which is true
Text Solution
|
- Which of the following are not the characteristics of crystalline soli...
Text Solution
|
- Choose the correct statements out of the following
Text Solution
|
- Which of the following statements are correct ?
Text Solution
|
- The corrent statement regarding defects is solids in solids is
Text Solution
|
- Diamond is:
Text Solution
|
- Select the correct statements (s)
Text Solution
|
- What is true about a bcc unit cell?
Text Solution
|
- Which of the following statements is (are) correct ?
Text Solution
|
- Which of the following statements is/are consistent with the propertie...
Text Solution
|