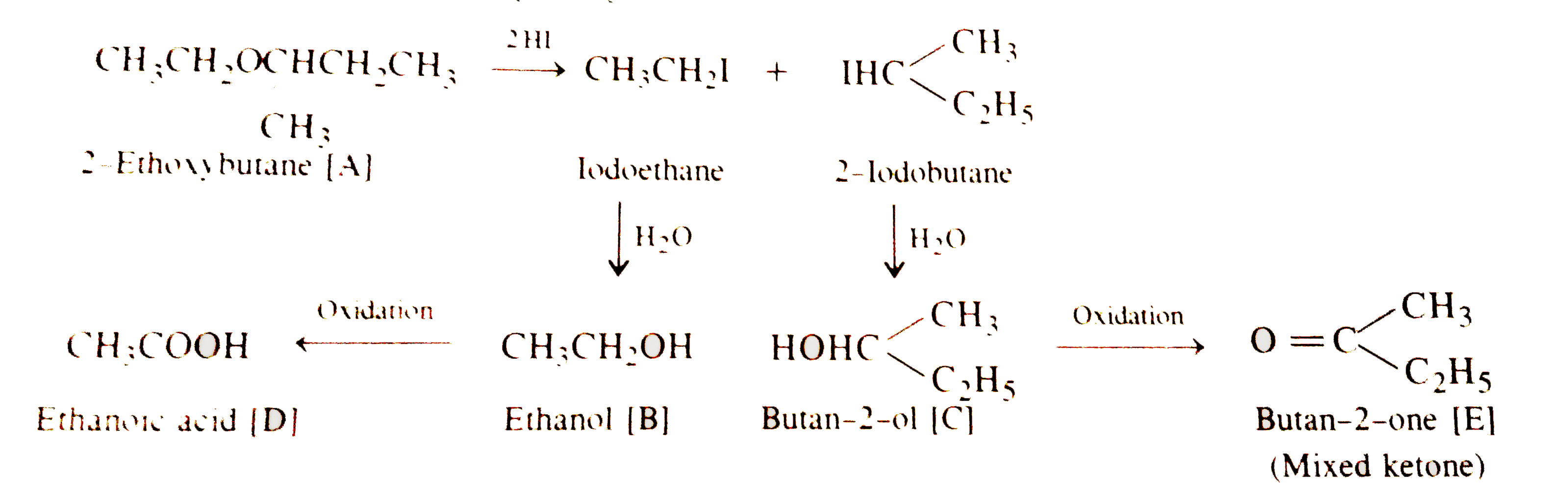Text Solution
Verified by Experts
Topper's Solved these Questions
ETHERS
DINESH PUBLICATION|Exercise N.C.E.R.T. IN TEXT QUESTIONS|3 VideosETHERS
DINESH PUBLICATION|Exercise N.C.E.R.T. EXERCISE|8 VideosELECTROCHEMISTRY
DINESH PUBLICATION|Exercise ADDITIONAL NUMERICAL PROBLEMS FOR PRACTICE|12 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
DINESH PUBLICATION|Exercise Brain Storming Multiple Chocie Questions|7 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ETHERS-(MCQs)
- An ether, (A) having molecular formula, C(6)H(14)O, when treated with ...
Text Solution
|
- Predict the product when alcohol undergoes dehydration with conc. H(2...
Text Solution
|
- Arrange the following alcohols in order of reactivity towards gaseous ...
Text Solution
|
- An alcohol (A) on heating with concentrated H(2)SO(4) gives alkene (B)...
Text Solution
|
- Consider the following reaction underset("Ester(x)")underset()(C(4)H...
Text Solution
|
- H(3)C-underset(CH(3))underset(|)overset(CH(3))overset(|)(C)-CH=CH(2) t...
Text Solution
|
- CH(3)-overset(CH(2)CH(3))overset(|)(CH)CH=CHCH(3)overset(H(3)O^(+))rar...
Text Solution
|
- The most effective pair of reagents for the preparation of tert bytyl ...
Text Solution
|
- Major product of the given reaction is CH(3)-underset(CH(3))underse...
Text Solution
|