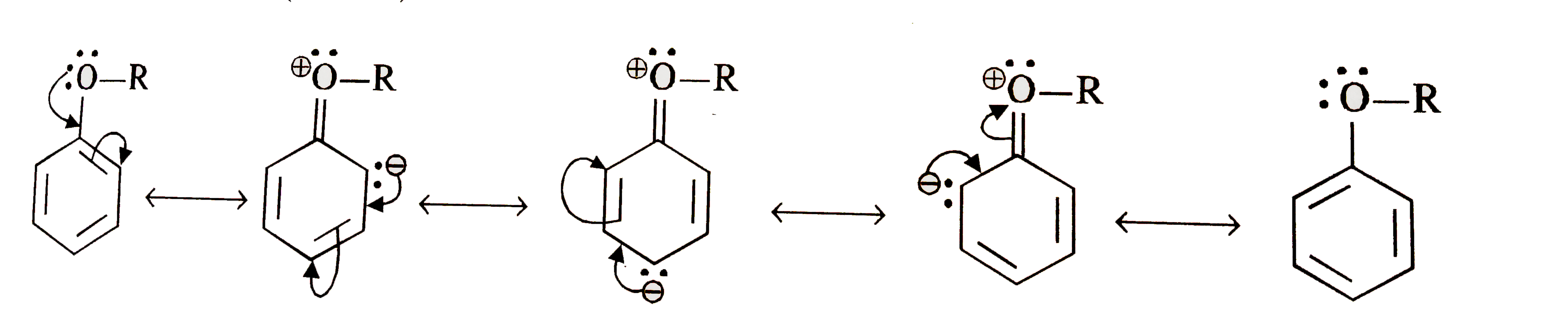
Text Solution
Verified by Experts
Topper's Solved these Questions
ETHERS
DINESH PUBLICATION|Exercise SHORT ANSWER TYPE QUESTIONS|35 VideosETHERS
DINESH PUBLICATION|Exercise LONG ANSWER TYPE QUESTIONS|4 VideosETHERS
DINESH PUBLICATION|Exercise N.C.E.R.T. IN TEXT QUESTIONS|3 VideosELECTROCHEMISTRY
DINESH PUBLICATION|Exercise ADDITIONAL NUMERICAL PROBLEMS FOR PRACTICE|12 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
DINESH PUBLICATION|Exercise Brain Storming Multiple Chocie Questions|7 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ETHERS-N.C.E.R.T. EXERCISE
- Explain the following with examples : (i) Williamson ether synthesi...
Text Solution
|
- Give the IUPAC names of the following ethers : (i) CH(3)OCH(2)-under...
Text Solution
|
- Write the names of the reagents and equations for the preparation of t...
Text Solution
|
- Illustrate with examples the limitations of Williamson's synthesis for...
Text Solution
|
- How is 1-propoxypropane synthesised from propan-1-ol ? Write mechanism...
Text Solution
|
- Write the equation for the reaction of HI with : (i) 1-Propoxypropan...
Text Solution
|
- Explain the fact that in alkyl aryl ethers, alkoxy group : (i) activ...
Text Solution
|
- Write the equations of the following reactions: i. Friedel-Crafts r...
Text Solution
|