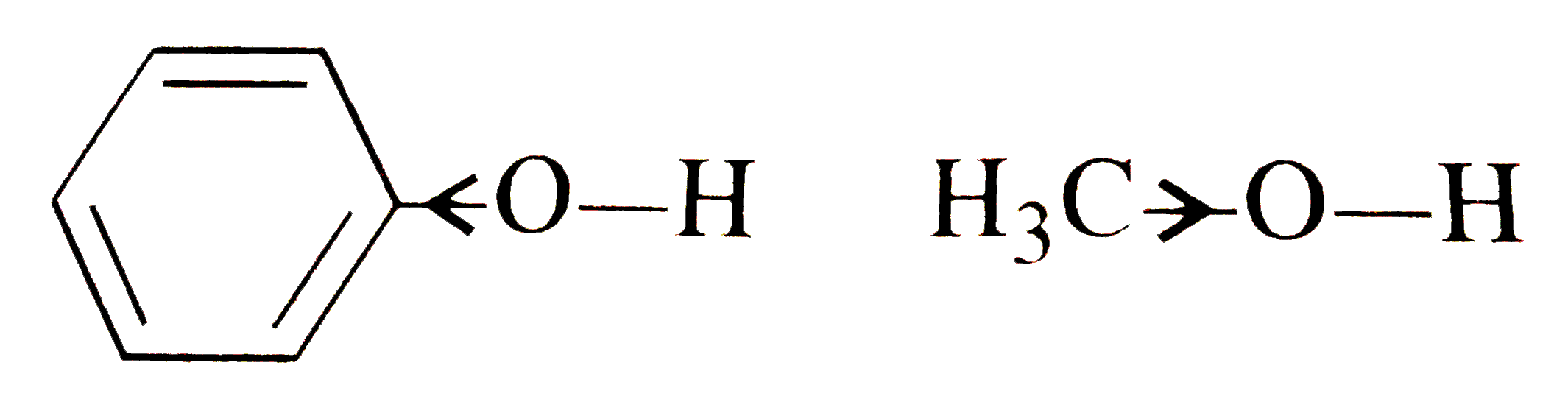Text Solution
Verified by Experts
Topper's Solved these Questions
ETHERS
DINESH PUBLICATION|Exercise LONG ANSWER TYPE QUESTIONS|4 VideosETHERS
DINESH PUBLICATION|Exercise ADDITIONAL IMPORTANT QUESTIONS|20 VideosETHERS
DINESH PUBLICATION|Exercise N.C.E.R.T. EXERCISE|8 VideosELECTROCHEMISTRY
DINESH PUBLICATION|Exercise ADDITIONAL NUMERICAL PROBLEMS FOR PRACTICE|12 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
DINESH PUBLICATION|Exercise Brain Storming Multiple Chocie Questions|7 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ETHERS-SHORT ANSWER TYPE QUESTIONS
- Arrange the following compounds in decreasing order of acidity. H(2)...
Text Solution
|
- Name the enzymes and write the reactions involved in the preparation o...
Text Solution
|
- How can propan-2-one be converted into tert-butyl alcohol ?
Text Solution
|
- Write the structures of the isomers of alcohols with molecular formula...
Text Solution
|
- Explain why is OH group in phenols more strongly held as compared to O...
Text Solution
|
- Explain why nucleophilic substitution reactions rae not very common in...
Text Solution
|
- Preparation of alcohols from alkenes involves the electrophilic attack...
Text Solution
|
- Explain why is O=C=O non polar while R-O-R is polar ?
Text Solution
|
- Why is the reactivity of all the three classes of alcohols with conc. ...
Text Solution
|
- Write steps to carry out the conversion of phenol to aspirin.
Text Solution
|
- Nitration is an example of aromatic electrophilic substitution and its...
Text Solution
|
- In Kolbe's reactio insteaded of phenol, phenoxide ion is treated with ...
Text Solution
|
- Dipole moment of phenol is smaller than that of methanol. Why ?
Text Solution
|
- Ethers can be prepared by Williamson synthesis in which an alkyl halid...
Text Solution
|
- Why is the C-O-H bond angle in alcohols slightly less than the tetrahe...
Text Solution
|
- Explain why low molecular mass alcohols are soluble in water ?
Text Solution
|
- Explain why p-nitrophenol is more acidic than phenol ?
Text Solution
|
- Explain why alcohols and ethers of comparable molecular mass have diff...
Text Solution
|
- The carbon-oxygen bond in phenol is slightly stronger than that in met...
Text Solution
|
- Arrange water, ethanol and phenol in increasing order of acidity and g...
Text Solution
|