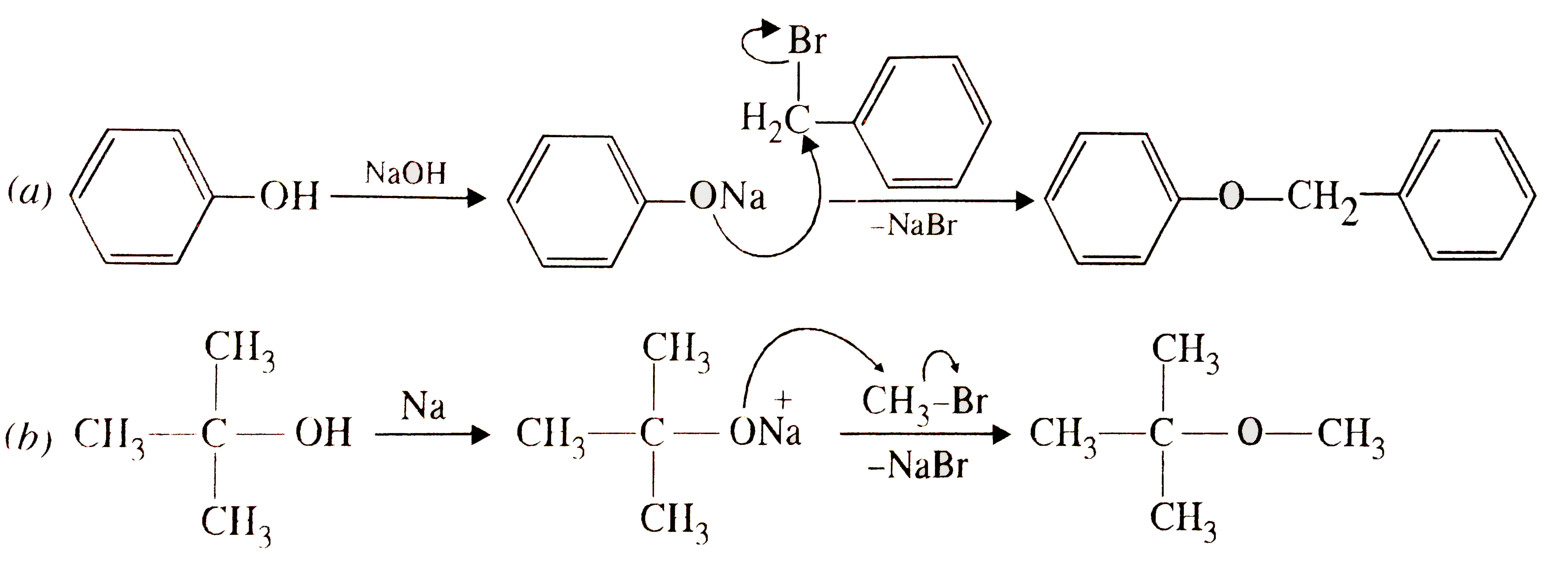
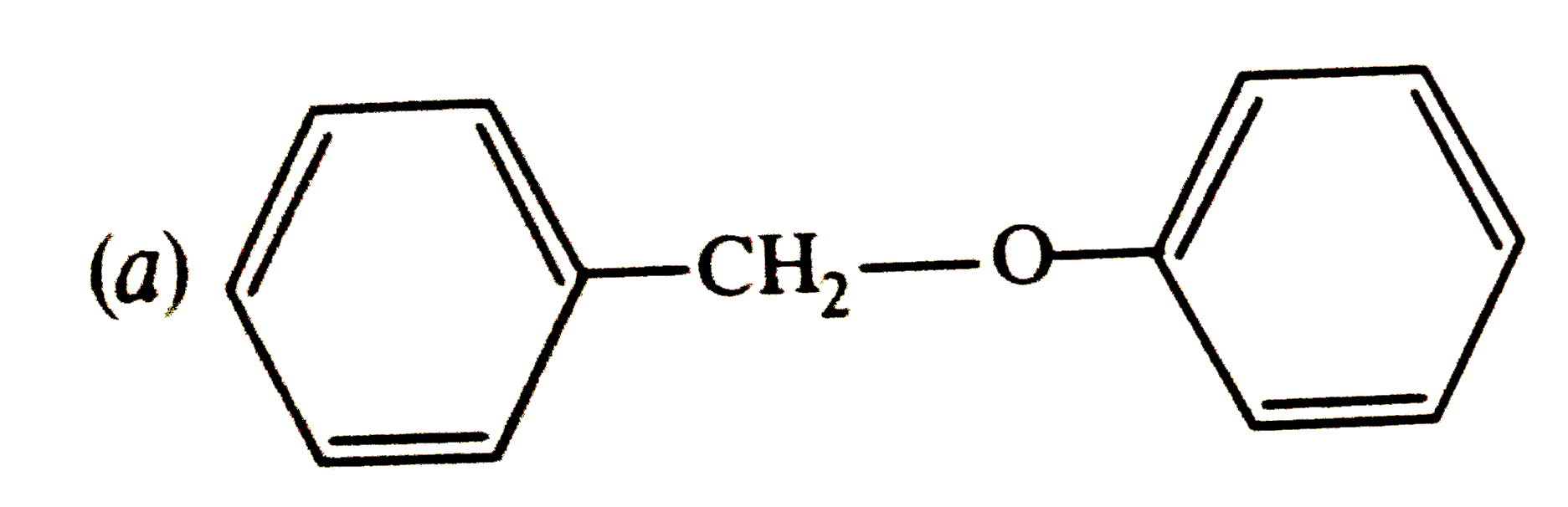Text Solution
Verified by Experts
Topper's Solved these Questions
ETHERS
DINESH PUBLICATION|Exercise QUESTIONS FROM BOARD EXAMINATIONS|17 VideosETHERS
DINESH PUBLICATION|Exercise HIGHER ORDER THINKING SKILLS (HOTS) QUESTIONS|6 VideosETHERS
DINESH PUBLICATION|Exercise LONG ANSWER TYPE QUESTIONS|4 VideosELECTROCHEMISTRY
DINESH PUBLICATION|Exercise ADDITIONAL NUMERICAL PROBLEMS FOR PRACTICE|12 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
DINESH PUBLICATION|Exercise Brain Storming Multiple Chocie Questions|7 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ETHERS-ADDITIONAL IMPORTANT QUESTIONS
- Ethers have less dipole moments than alcohols. Justify.
Text Solution
|
- Sodium metal can be used to dry diethyl ether and not ethyl alcoho. Wh...
Text Solution
|
- Why are ethers highly inflammale substances ?
Text Solution
|
- An ether possesses dipole moment even if the alkyl groups present in i...
Text Solution
|
- Dimethyl ether is completely soluble in water but diethyl ehter is sol...
Text Solution
|
- Ethers are relatively inert. Justify.
Text Solution
|
- why is it not possible to prepare ditertiary butyl ether by Williamson...
Text Solution
|
- With the help of Williamson's synthesis, prepare the following ethers....
Text Solution
|
- Why are secondary and tertiary alcohols not suitable for praparing eth...
Text Solution
|
- Methyl phenyl ether cannot be prepared from bromoenzene. Discuss.
Text Solution
|
- Phenyl methyl ether (on anisole) reacts with HI to give phenol and met...
Text Solution
|
- Ethers have low solubility in water but high solubility in conc. H(2)S...
Text Solution
|
- Why is diethyl ether used as solvent in the prparation of Grignard rea...
Text Solution
|
- Sometimes explosionn occurs durring the distilliation of an ether Expl...
Text Solution
|
- Ethers are cleaved by acids and not by bases. Explain.
Text Solution
|
- 2,2-Dimethyloxirane can be cleaved by acid (H^(o+)). Write the mechani...
Text Solution
|
- Which of the following is the correct method for synthesising methyl-t...
Text Solution
|
- Which product is formed when is reacted with HI ?
Text Solution
|
- Complete the following : (CH(3))(3)CBr+alc.KOHrarr[A]overset(HOCI)...
Text Solution
|
- Give the necessary steps involved in the conversion of benzene to 1,2-...
Text Solution
|