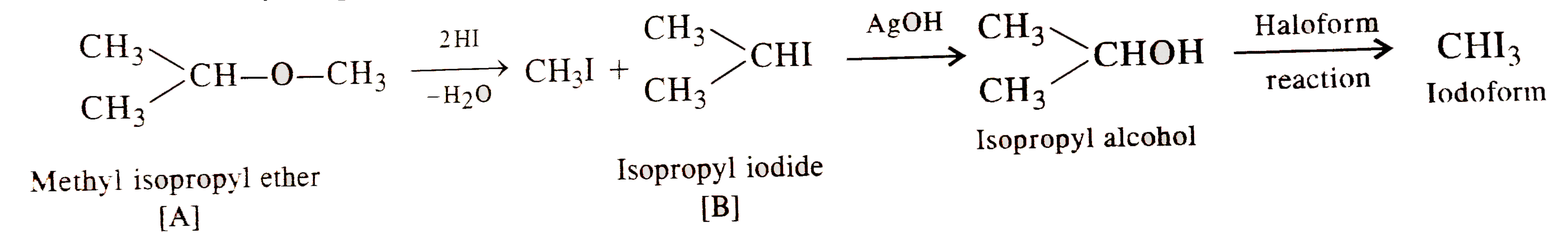Text Solution
Verified by Experts
Topper's Solved these Questions
ETHERS
DINESH PUBLICATION|Exercise MULTIPLE CHOICE QUESTIONS (TYPE-I)|16 VideosETHERS
DINESH PUBLICATION|Exercise MULTIPLE CHOICE QUESTIONS (TYPE-II)|5 VideosETHERS
DINESH PUBLICATION|Exercise QUESTIONS FROM BOARD EXAMINATIONS|17 VideosELECTROCHEMISTRY
DINESH PUBLICATION|Exercise ADDITIONAL NUMERICAL PROBLEMS FOR PRACTICE|12 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
DINESH PUBLICATION|Exercise Brain Storming Multiple Chocie Questions|7 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ETHERS-HIGHER ORDER THINKING SKILLS (HOTS) QUESTIONS
- Two compounds [A] and [B] have molecular formula C(2)H(6)O. On reactin...
Text Solution
|
- A neutral compound (A) having C,H and O, on refluxing with HI yields m...
Text Solution
|
- When aqueous HI reacts with methoxyethane, methyl iodide and ethanol a...
Text Solution
|
- Compound (A) C(4)H(10)O, is found to be soluble in sulphuric acid . (...
Text Solution
|
- An organic compound A (C(2)H(6)O) reacts with sodium to form a compoun...
Text Solution
|
- Write the strucrtures of the products. (CH(3))(2)CH-OCH(3)underset("he...
Text Solution
|