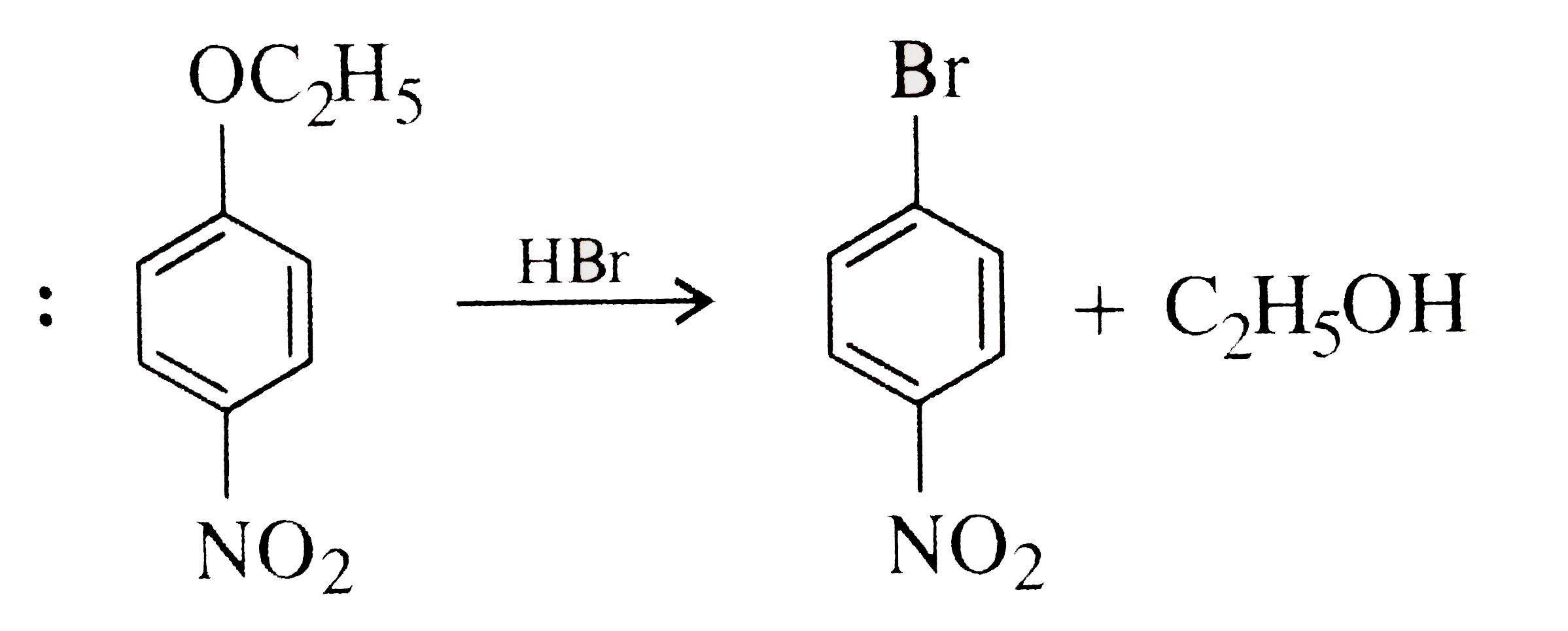A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ETHERS
DINESH PUBLICATION|Exercise INTEGER ANSWER TYPE QUESTIONS|7 VideosETHERS
DINESH PUBLICATION|Exercise (MCQs)|8 VideosETHERS
DINESH PUBLICATION|Exercise JEE (MAIN) & OTHER ENGINEERING ENTRANCE EXAMINATIONS|17 VideosELECTROCHEMISTRY
DINESH PUBLICATION|Exercise ADDITIONAL NUMERICAL PROBLEMS FOR PRACTICE|12 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
DINESH PUBLICATION|Exercise Brain Storming Multiple Chocie Questions|7 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ETHERS-ASSERTION -REASON TYPE QUESTIONS
- Assertion: Alcohols have higher boiling points than ethers of comparab...
Text Solution
|
- Assertion : Ethers have specific dipole moment values Reason : The C...
Text Solution
|
- (A) With HI, anisole forms iodobenzene and methyl alcohol. (R ) I^(-...
Text Solution
|
- Assertion : Ethers behave as bases in the presence of mineral acids. ...
Text Solution
|
- Asssertion : (CH(3))(3) COH when heated with conc. H(2)SO(4) gives iso...
Text Solution
|
- Assertion : Tert- butyl methyl ether on cleavage with HI at 373 K give...
Text Solution
|
- Asssertion : 2-Bromobutane on reacting with sodium ehtoxide. In ethano...
Text Solution
|
- Assertion : The major product formed by heating C(6)H(5)CH(2)OCH(3) wi...
Text Solution
|
- Cleavage of anisole with HI at 373 K gives phenol and CH(3)I Reason ...
Text Solution
|
- Phenol is more reactive than benzene towards electrophilic substitutio...
Text Solution
|
- Assertion : Phenol undergoes Kolbe's reaction whereas ethanol does ...
Text Solution
|
- Assertion: The pK(a) of acetic acid is lower than that of phenol. R...
Text Solution
|
- Assertion: Phenol forms 2,4,6-tribromophenol on treatement with Br(2) ...
Text Solution
|
- Assertion : Reason : Alkyl arly ethers on reaction with halogen aci...
Text Solution
|