Text Solution
Verified by Experts
Topper's Solved these Questions
CARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise SOME TYPICAL WORD PROBLEMS BASED ON CONVERSION|7 VideosCARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise N.C.E.R.T. EXMPLAR PROBLEMS|22 VideosCARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise QUESTIONS FROM BOARD EXAMINATIONS|67 VideosBIOMOLECULES
DINESH PUBLICATION|Exercise MATRIX - MATCH TYPE|8 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Unit Test - 1|20 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CARBOXYLIC ACIDS -HIGHER ORDER THINKING SKILLS (HOTS) QUESTIONS
- Dissociation constants of benzoic acid, p-nitrobenzoic acid and p-hydr...
Text Solution
|
- Two methods have been adopted to prepare 2-methylbutanoic acid from bu...
Text Solution
|
- How will you prepare each of the following carboxylic acids by using a...
Text Solution
|
- Give steps involved in the conversion of : (a) Toluene to prop-bromo...
Text Solution
|
- An ester has a molecular mass of 102. On aqueous hydrolysis, it produc...
Text Solution
|
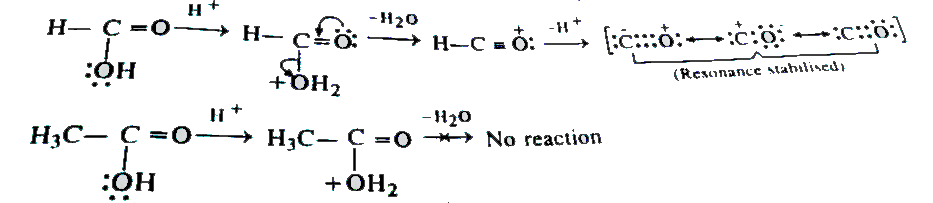
- HCOOH is dehydrated when warmed with conc. H(2)SO(4) but CH(3)COOH is ...
Text Solution
|
- Predict the product Q of the following reaction :
Text Solution
|