Text Solution
Verified by Experts
Topper's Solved these Questions
CARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise N.C.E.R.T. EXMPLAR PROBLEMS|22 VideosCARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise ASSERTION-REASON TYPE QUESTIONS|5 VideosCARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise HIGHER ORDER THINKING SKILLS (HOTS) QUESTIONS|7 VideosBIOMOLECULES
DINESH PUBLICATION|Exercise MATRIX - MATCH TYPE|8 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Unit Test - 1|20 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CARBOXYLIC ACIDS -SOME TYPICAL WORD PROBLEMS BASED ON CONVERSION
- Compound (A) (C(6)H(12)O(2)) on reduction with LiAlH(4) yielded two co...
Text Solution
|
- An organic compound A (C(7)H(6)Cl(2)) on treatment with NaOH solution ...
Text Solution
|
- Identify A, B, C, D and E in the following sequence of reactions.
Text Solution
|
- An acid [A] C(8)H(7)O(2)Br on bromination in the presence of FeBr(3) g...
Text Solution
|
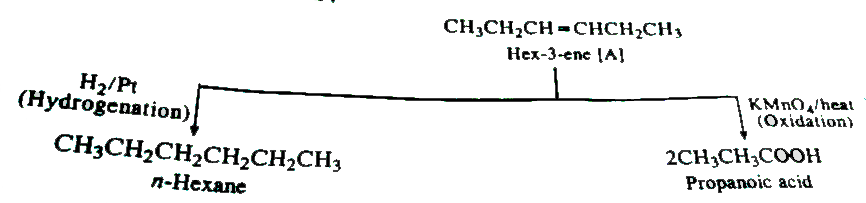
- The hydrocarbon [A] adds one mole of hydrogen in the presence of a pla...
Text Solution
|
- An organic compound (A) (molecular formula C(8)H(16)O(2)) was hydrolys...
Text Solution
|
- Write the structures of A, B, C and D in the following reactions :
Text Solution
|