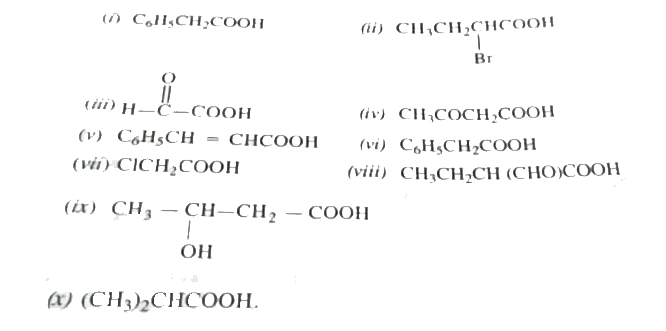
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise MULTIPLE CHOICE QUESTION BANK (MCQB)|113 VideosCARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise MATRIX-MATCH TYPE QUESTIONS|5 VideosCARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise ASSERTION-REASON TYPE QUESTIONS|5 VideosBIOMOLECULES
DINESH PUBLICATION|Exercise MATRIX - MATCH TYPE|8 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Unit Test - 1|20 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CARBOXYLIC ACIDS -ASSIGNMENT
- Write the IUPAC name of
Text Solution
|
- Write the structural formulae and give IUPAC names of the following : ...
Text Solution
|
- What is the relation between K(a)" and "pK(a) value of carboxylic acid...
Text Solution
|
- Acetic acids exists in dimer state in benzene due to
Text Solution
|
- Why is benzoic acid a stronger acid than acetic acid ?
Text Solution
|
- The product formed during Hell-Volhard-Zelinsky reaction is
Text Solution
|
- Arrange the following in decreasing order of boiling point. (i) CH(3...
Text Solution
|
- Explain the following about acetic acid (i) Its boiling point is hig...
Text Solution
|
- What happens when : (i) Sodium acetate is heated with soda lime (i...
Text Solution
|
- Complete the following : (i) CH(3)COOHoverset(P//B(2))rarr , (ii) CH...
Text Solution
|
- Arrange the following in decreasing order of acidic strength
Text Solution
|
- How will your prepare teriary butyl alcohol from acetic acid ?
Text Solution
|
- Complete the missing links in the following :
Text Solution
|
- (a) How will you prepare benzoic acid (i) from ethyl benzene (ii) ...
Text Solution
|
- Explain giving reasons each of the following : (i) Chloroacetic acid...
Text Solution
|
- Write short notes on : (i) Kolbe's electrolysis (ii) H.V.Z. reaction...
Text Solution
|
- How will your convert : (i) Propionic acid to acetic acid (ii) Acety...
Text Solution
|
- Give chemical tests to distinguish between : (i) Benzoic acid and ph...
Text Solution
|
- Justify : Trichloroacetic acid is a stronger acid than dichloroaceti...
Text Solution
|
- (a) Describe the preparation of acetic acid from acetylene. How can th...
Text Solution
|