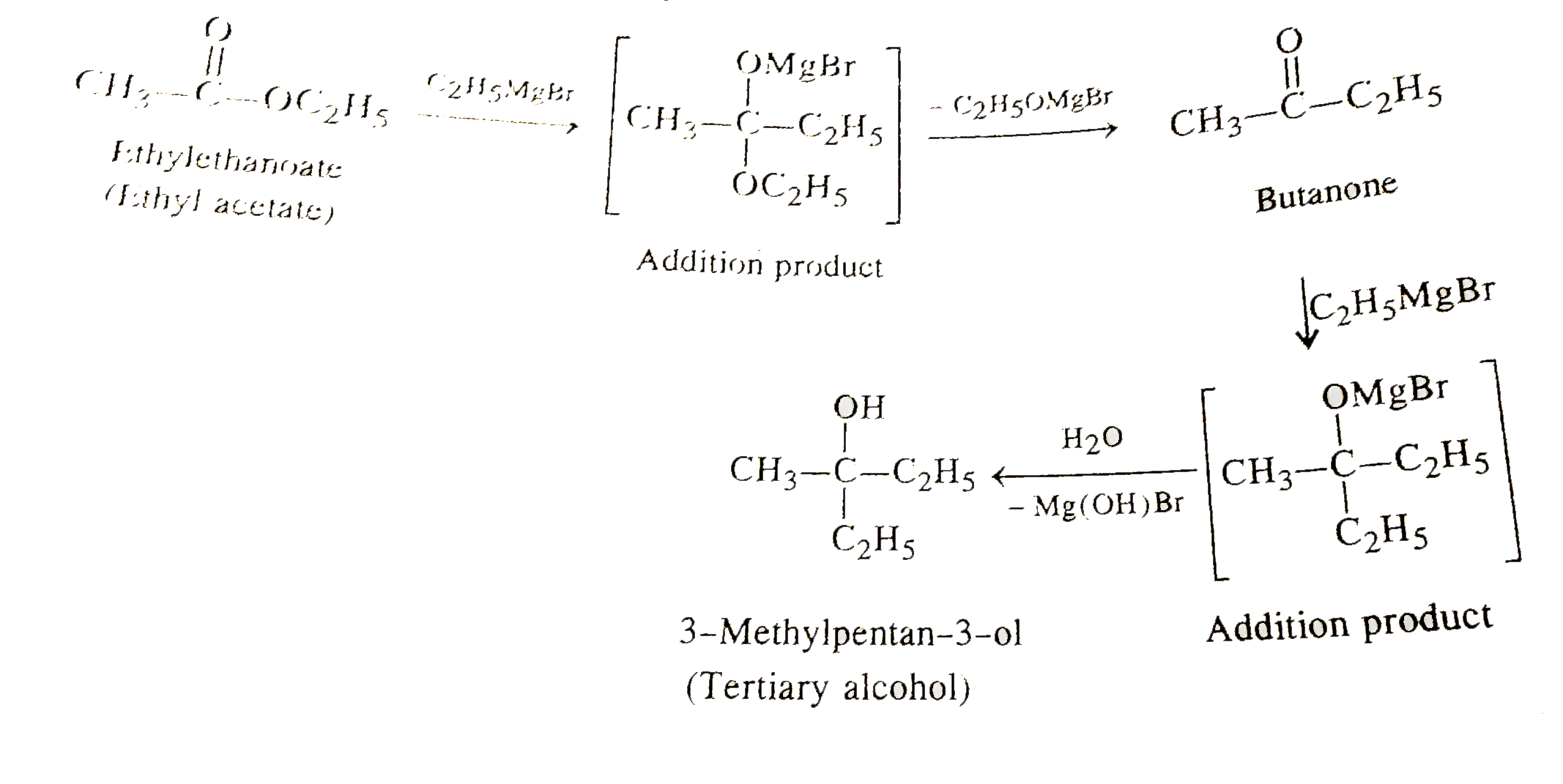Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ALCOHOLS AND PHENOLS-Matrix
- With the help of chemical equations, write the structural formula of t...
Text Solution
|
- Match the statement (A, B, C, D) in column I with statement (p, q, r, ...
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the chemical conversions in Column I with the appropriate reagen...
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
Text Solution
|
- Match the List I with List II and select the correct answer using the ...
Text Solution
|
- Match the List I with List II and select the correct answer using the ...
Text Solution
|