Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOLS AND PHENOLS
DINESH PUBLICATION|Exercise NCERT Exercise|23 VideosALCOHOLS AND PHENOLS
DINESH PUBLICATION|Exercise ADDITIONAL IMPORTANT QUESTION|30 VideosALCOHOLS AND PHENOLS
DINESH PUBLICATION|Exercise COMPLETION QUESTION|2 VideosALDEHYDES AND KETONES
DINESH PUBLICATION|Exercise Interger|5 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ALCOHOLS AND PHENOLS-NCERT In-Text Questions
- Classify the following into primary, secondary and tertiary alcohols ...
Text Solution
|
- Identify allylic alcohol in the above examples.
Text Solution
|
- Give the IUPAC names of the following compounds : (i) {:(" ...
Text Solution
|
- Shown how the following alcohols can be prepared by the action of suit...
Text Solution
|
- Write the structures of the products of the following reactions : (i...
Text Solution
|
- Give structures of the products you would expect when each of the foll...
Text Solution
|
- Predict the major product of acid catalysed dehydration of : (i) 1-M...
Text Solution
|
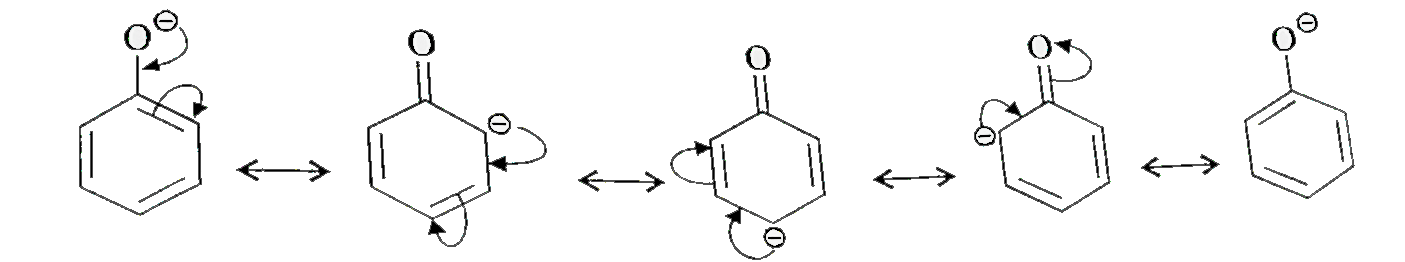
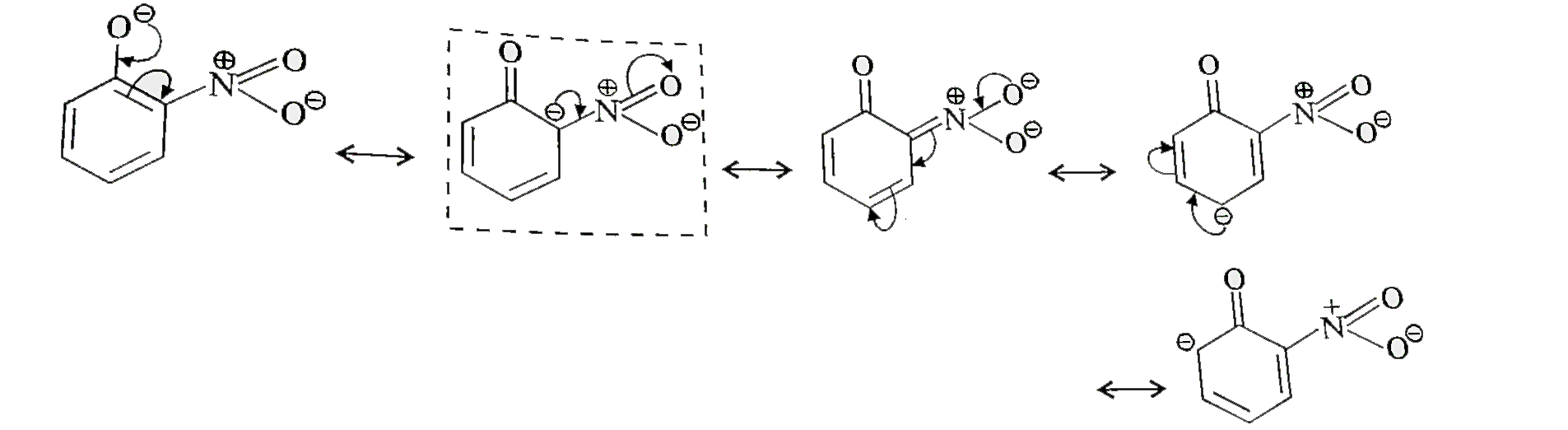
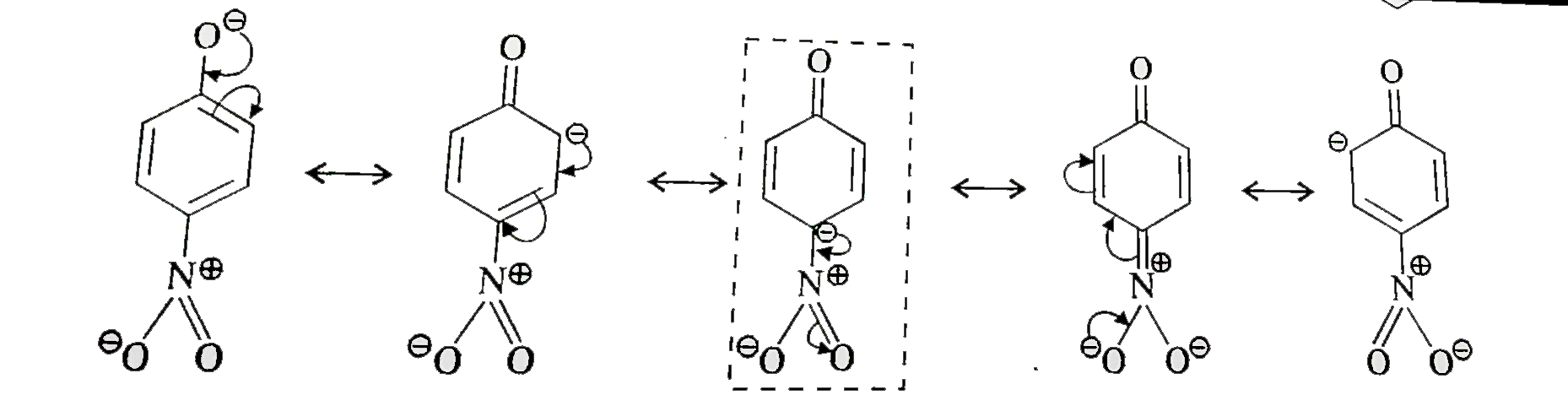
- Ortho and para nitrophenols are more acidic than phenol. Draw the reso...
Text Solution
|
- Explain the following with an example: i. Kolbe's reaction ii. R...
Text Solution
|