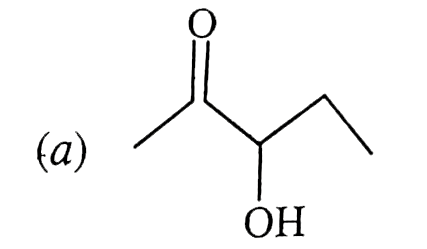
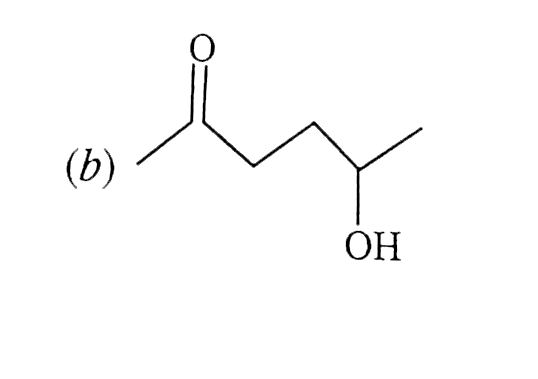
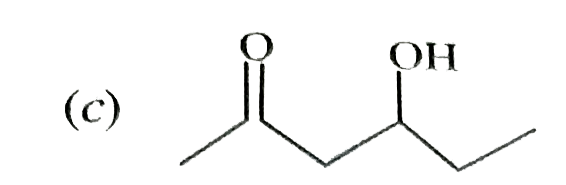
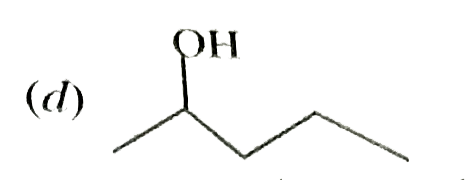
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ALCOHOLS AND PHENOLS-MCQB
- In the following reaction H(3)C-underset(CH(3))underset(|)overset(CH...
Text Solution
|
- What amount of bromine will be required to convert 2g of phenol into 2...
Text Solution
|
- Which of the following will be most readily dehydrated in acidic condi...
Text Solution
|
- Among the following sets of reactants which one produces anisole?
Text Solution
|
- An ether solution of PhCH(3) (I), PhNH(2) (II) and PhOH (III) is extra...
Text Solution
|
- Reaction of phenol with chloroform in presence of dilute sodium hydrox...
Text Solution
|
- Which of the following is not the product of hydration of
Text Solution
|
- Dehydration of which one of the following alcohols produces an alkene ...
Text Solution
|
- X, Y and Z are
Text Solution
|
- Out of the following compounds which produce CO(2) gas when treated wi...
Text Solution
|
- Out of following compounds, which will give iodoform test ? (i) Isopro...
Text Solution
|
- Which of the following reagents would distinguish cis cyclopenta-1,2- ...
Text Solution
|
- Which one of the following -compounds does not react with nitrous acid...
Text Solution
|
- Which of the following compounds contain at least one secondary alcoho...
Text Solution
|
- Which one is the most acidic compound?
Text Solution
|
- Identify the major product P, Q and R in the following sequence of rea...
Text Solution
|
- In the reaction the electrophile involved is
Text Solution
|
- The finalproduct of the reaction,
Text Solution
|
- In Reimer-Timann reaction, the reagent used is
Text Solution
|
- Arrange the following gem diols in decreasing order of stability :
Text Solution
|