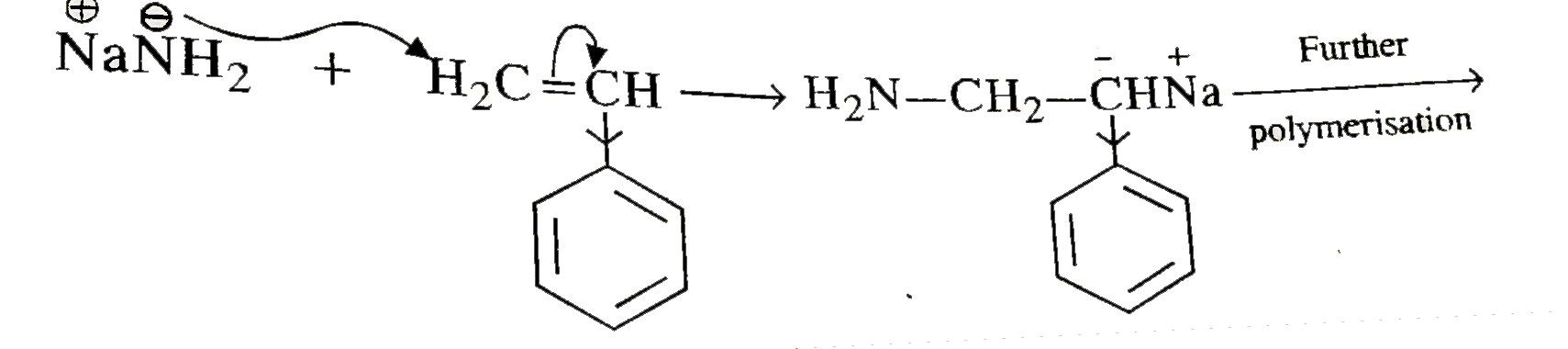Text Solution
Verified by Experts
Topper's Solved these Questions
POLYMERS
DINESH PUBLICATION|Exercise Question from Board examinations|62 VideosPOLYMERS
DINESH PUBLICATION|Exercise Assignment|72 VideosPOLYMERS
DINESH PUBLICATION|Exercise Long answer type question|5 VideosORGANIC COMPOUNDS WITH FUNCTIONAL GROUP CONTAINING HALOGENS
DINESH PUBLICATION|Exercise UNIT TEST - 5|37 VideosPRINCIPLES RELATED TO PRACTICAL CHEMISTRY
DINESH PUBLICATION|Exercise Brain teasers-42|1 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-POLYMERS-Additional important question
- Name a substance which inhibits free radical polymerisation.
Text Solution
|
- Nylon -66 is
Text Solution
|
- What is mode of free radical polymerisation in alkenes ?
Text Solution
|
- Write the structures of the monomers used for getting the following po...
Text Solution
|
- Why should the monomer used in addition polymerisation through free r...
Text Solution
|
- How does the presence of carbon tetrachloride influence the course of ...
Text Solution
|
- Explain how does 1, 3-butadiene polymerise by different route
Text Solution
|
- Why does styrene undergo anionic polymerisation easily ?
Text Solution
|
- Why is cationic polymerisation preferred in case of vinylic monomers c...
Text Solution
|
- Depict a free radical mode of addition polymerisation in isoprene.
Text Solution
|
- Why are the number 66 and 6 put in the names of nylon 66 and nylon 6 ?
Text Solution
|
- What is the difference between thermosetthing and thermosplastic polym...
Text Solution
|
- How is bakelite formed ? Explains the reactions with equation
Text Solution
|
- Polymers are always macromolecules but macromolecules are not always p...
Text Solution
|
- Can a co-polymer be formed both in additin and condensation polymerisa...
Text Solution
|
- Explain how does 1, 3-butadiene polymerise by different route
Text Solution
|
- Are polyesters and polyacrylates same ? Justify your answer.
Text Solution
|
- Will you prefer to polymerise acrylonitrile under anionic or cationic ...
Text Solution
|