A
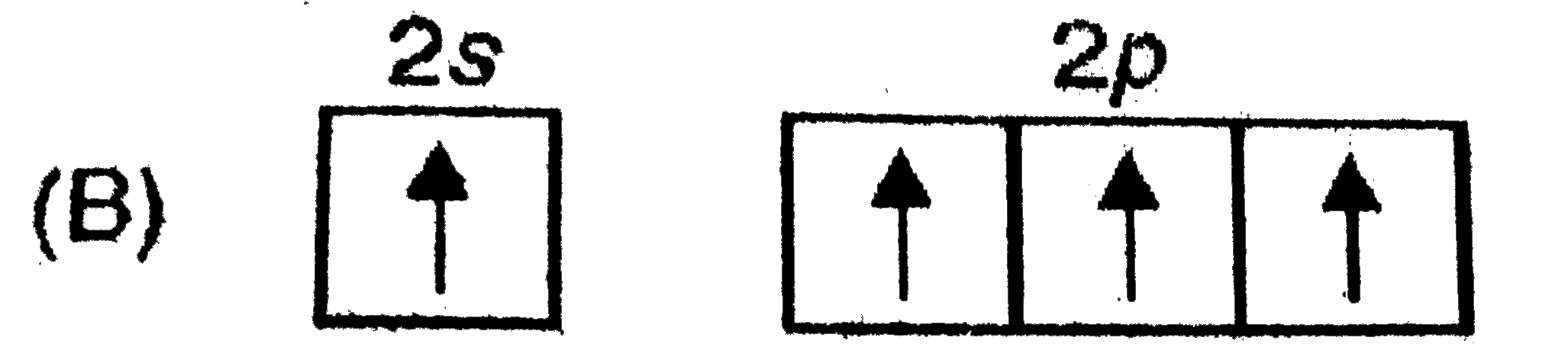
B
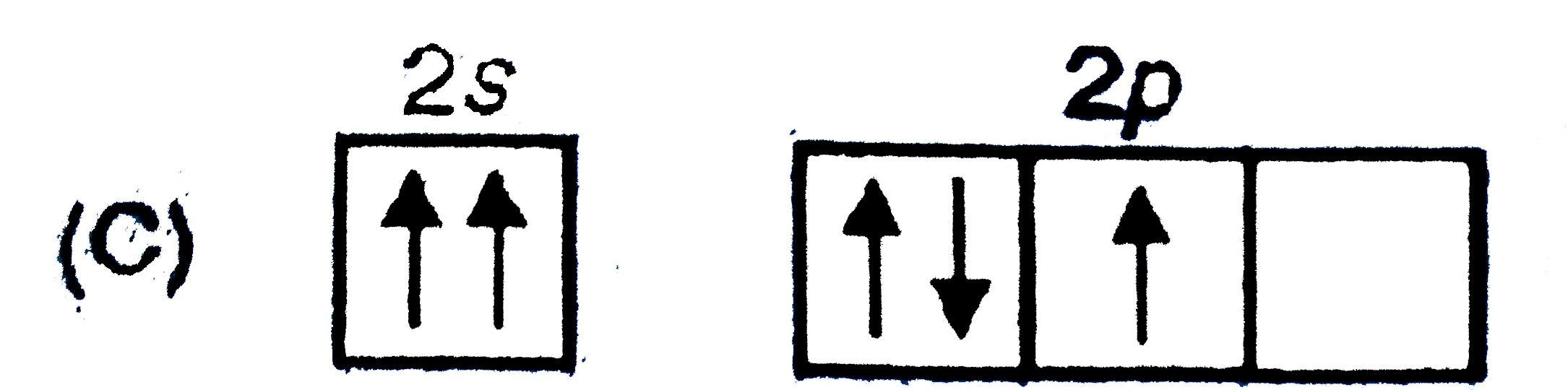
C
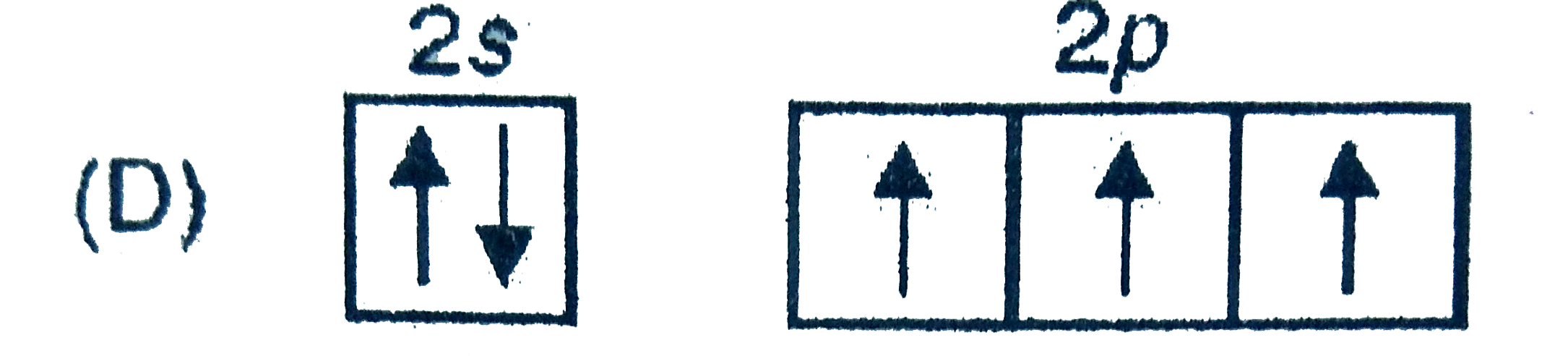
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ATOMIC STRUCTURE-Ultimate Preparatory Package
- In which of the following electron distribution in ground state, only ...
Text Solution
|
- Pick out of the correct statement: In a a cathode ray tube cathode r...
Text Solution
|
- In a discharged tube containing only one elementary gas other than hyd...
Text Solution
|
- Pick out the correct statement : In a multielectron atom
Text Solution
|
- Out of the physical, chemical and nuclear properties of an element, wh...
Text Solution
|
- Pick up the correct statement about the following pairs I : Ar and S...
Text Solution
|
- A 3p-orbital has
Text Solution
|
- 1.00 cm^(-1) represents
Text Solution
|
- Which of the following relates to light as wave motion as well as a st...
Text Solution
|
- Light of wavelength lambda shines on a metal surface with initail X an...
Text Solution
|
- Light of wavelength lambda shines on a metal surface with initail X an...
Text Solution
|
- Number of possible orbital diagrams for the configurate 1s^(2) 2p^(1) ...
Text Solution
|
- Pick out of the correct statement about de Broglie concept
Text Solution
|
- Which d-orbtial has its four lobes along the axis?
Text Solution
|
- Which d-orbital does not have four lobes?
Text Solution
|
- Which quantum number is not related with Schrodinger equation :-
Text Solution
|