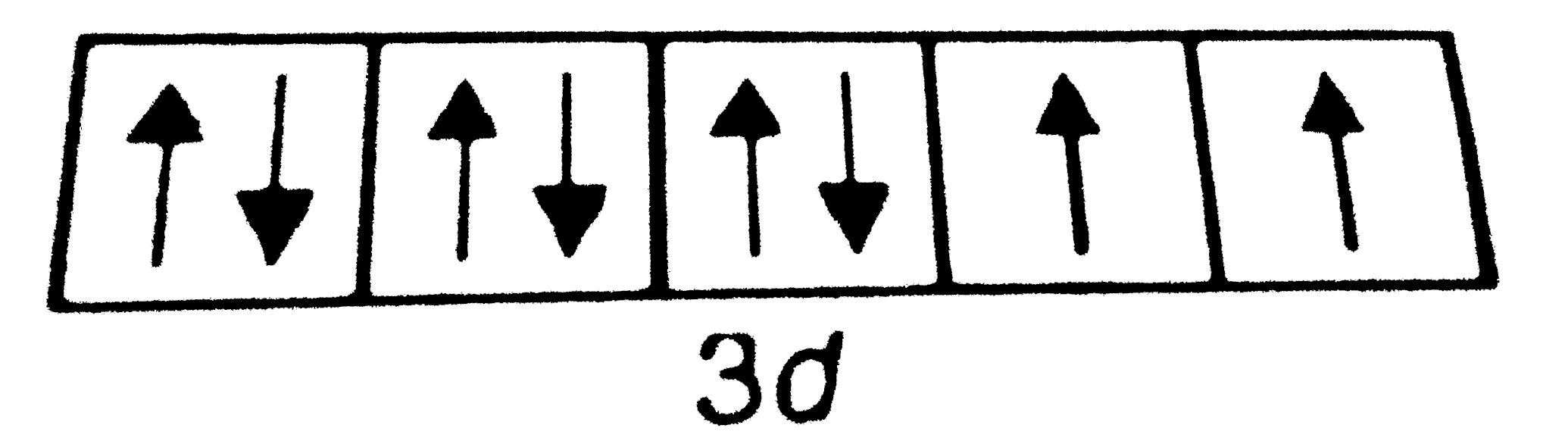
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
DINESH PUBLICATION|Exercise Compre.|17 VideosATOMIC STRUCTURE
DINESH PUBLICATION|Exercise Matrix match type MCQs|5 VideosATOMIC STRUCTURE
DINESH PUBLICATION|Exercise Revision Questions|258 VideosAPPENDIX
DINESH PUBLICATION|Exercise Completion Reactions|15 VideosBIOMOLECULES
DINESH PUBLICATION|Exercise Reason|1 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ATOMIC STRUCTURE-Selected Straight Objective Type MCQs
- Which of the following statement (s) are (are) correct?
Text Solution
|
- The less ground state electronic configeration of nitrogen atom can...
Text Solution
|
- How many unpaired electrons are there in Ni^(2+)?
Text Solution
|
- Rutherford's experiment on the scattering of alpha particle showed for...
Text Solution
|
- The principal quantum number of an atom is related in the
Text Solution
|
- Any p arbital can accommodate up to
Text Solution
|
- The magnetic quantum number of an atom is releted to the
Text Solution
|
- Rutherford's scattering experiment is related to the size of the
Text Solution
|
- When alpha particle are sent through a thin metal foil ,most of the...
Text Solution
|
- Which electronic level would allow the hydrogen atom to absorb a phot...
Text Solution
|
- Bohr's model can explain
Text Solution
|
- Electromagnetic radiation with maximum wavelengths is :
Text Solution
|
- Rutherford's alpha particle scattering experiment eventually led to th...
Text Solution
|
- Which of the following sets of quantum numbers represents an imposs...
Text Solution
|
- The triad of nuclei that is isotonic is
Text Solution
|
- The orbital diagram in which the Aufbau principle is violated is
Text Solution
|
- The quantum number not obtained from the schrodinger's wave equation i...
Text Solution
|
- The outer shell configuration of the most electronegative element is
Text Solution
|
- If the speed of electron in the first bohr orbit of hydrogen atom is x...
Text Solution
|
- If wavelength of photon is 2.2 xx 10^(-11)m, h = 6.6 xx 10^(-34) Js, t...
Text Solution
|