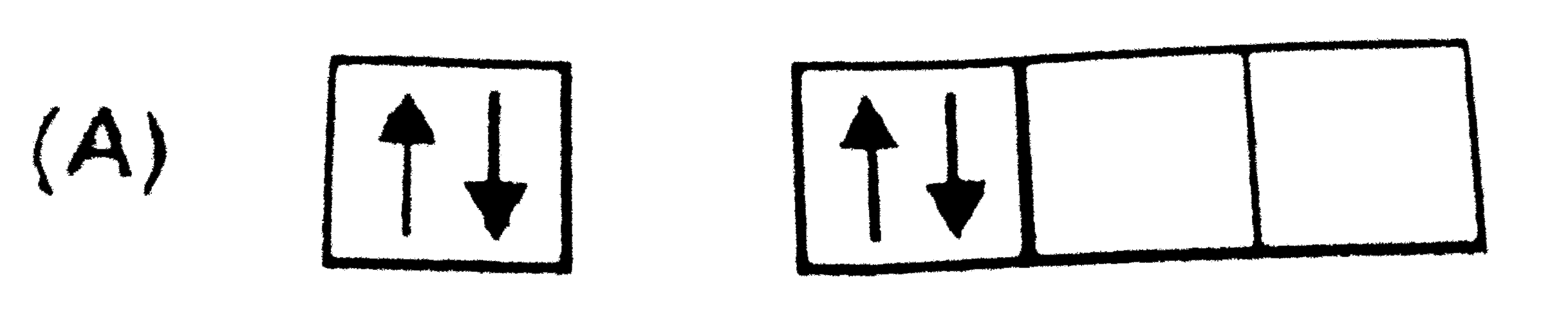
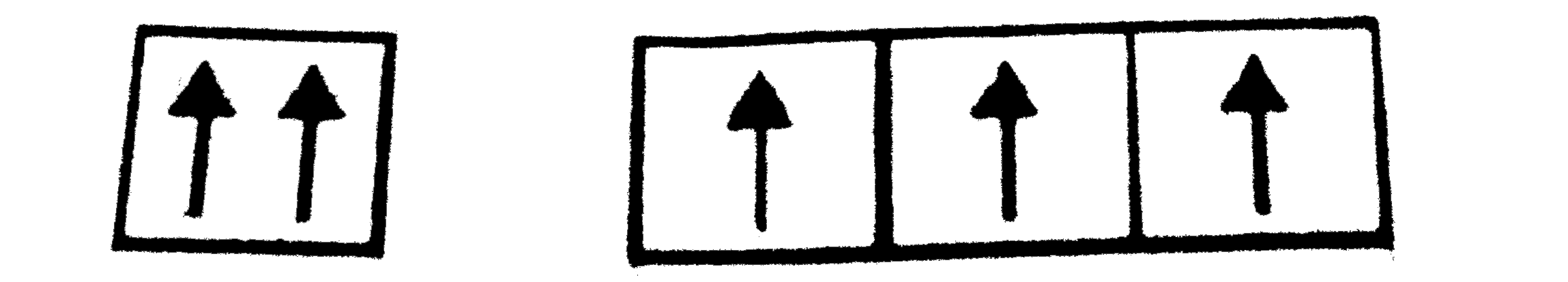
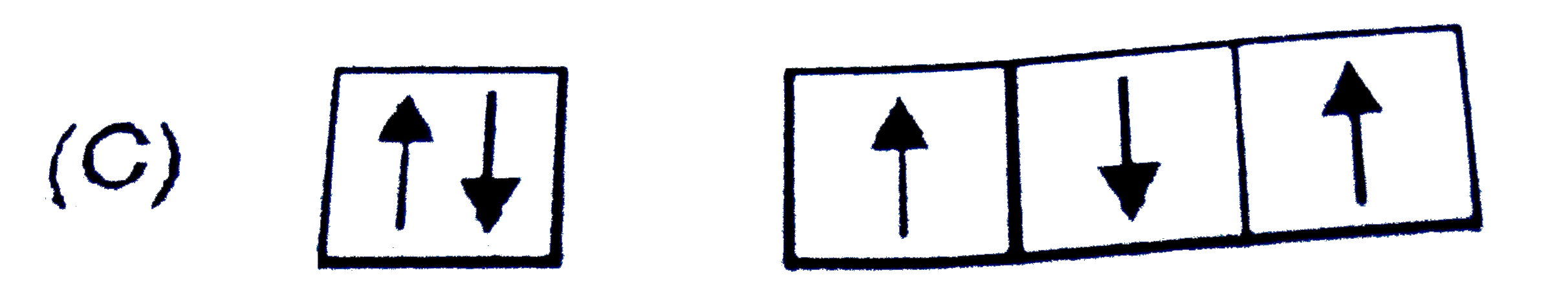
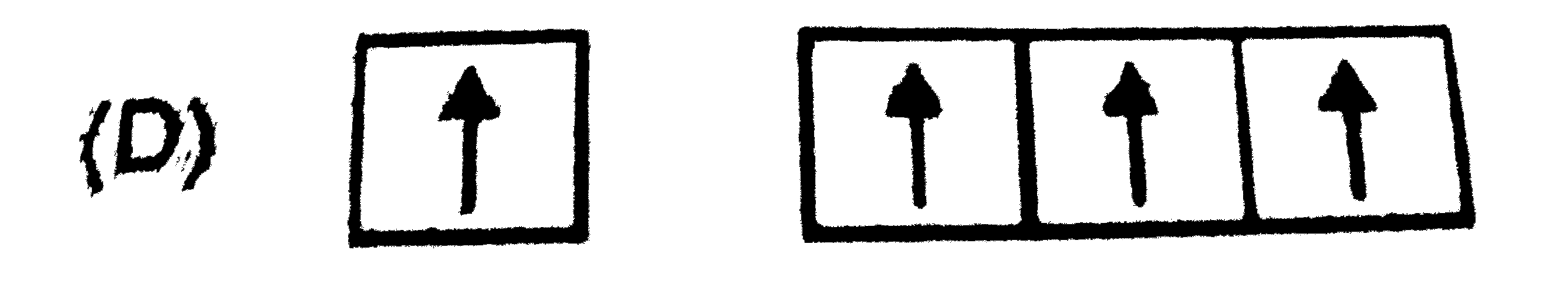
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
DINESH PUBLICATION|Exercise Compre.|17 VideosATOMIC STRUCTURE
DINESH PUBLICATION|Exercise Matrix match type MCQs|5 VideosATOMIC STRUCTURE
DINESH PUBLICATION|Exercise Revision Questions|258 VideosAPPENDIX
DINESH PUBLICATION|Exercise Completion Reactions|15 VideosBIOMOLECULES
DINESH PUBLICATION|Exercise Reason|1 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ATOMIC STRUCTURE-Selected Straight Objective Type MCQs
- What transition in the hydrogen spectrum would have the same wavelen...
Text Solution
|
- Pick out the isoelectronic structures from the following (i) CH(3)^(...
Text Solution
|
- Which of the following is a violation of the Pauli exclusion principle...
Text Solution
|
- From the given sets of quantum numbers, the one that is inconsistent w...
Text Solution
|
- The orbital angular momentum of an electron in 2s-orbital is
Text Solution
|
- Which of the following has maximum number of unpaired electron ?
Text Solution
|
- An element M has an atomic mass 19 and atomic number 9. Its ion is rep...
Text Solution
|
- The energy of an electron in the first Bohr orbit of H atom is -13.6 e...
Text Solution
|
- The electronic, identified by quantum numbers n and l, (i) n = 4, l = ...
Text Solution
|
- The electronic configuration of an element is 1s^(2)2s^(2)2p^(6)3s^(2)...
Text Solution
|
- The number of nodal planes in a p(x) orbital is :
Text Solution
|
- The wavelength associated with a gold weighing 200g and moving at a sp...
Text Solution
|
- The quatum numbers +(1)/(2) and -(1)/(2) for the electron spin represe...
Text Solution
|
- If nitrogen atoms had el,ectonic configuration is ? It would have en...
Text Solution
|
- Rutherford's scattering experiment, which established the nuclear mode...
Text Solution
|
- Identify the least stable among the following
Text Solution
|
- How many structures of F is possible ?
Text Solution
|
- The radius of which of the following orbit is same as that of the firs...
Text Solution
|
- The number of radial nodes in 3s and 2p, respectively, are
Text Solution
|
- A proton is about 1840 times heavier than an electron. When it is acce...
Text Solution
|