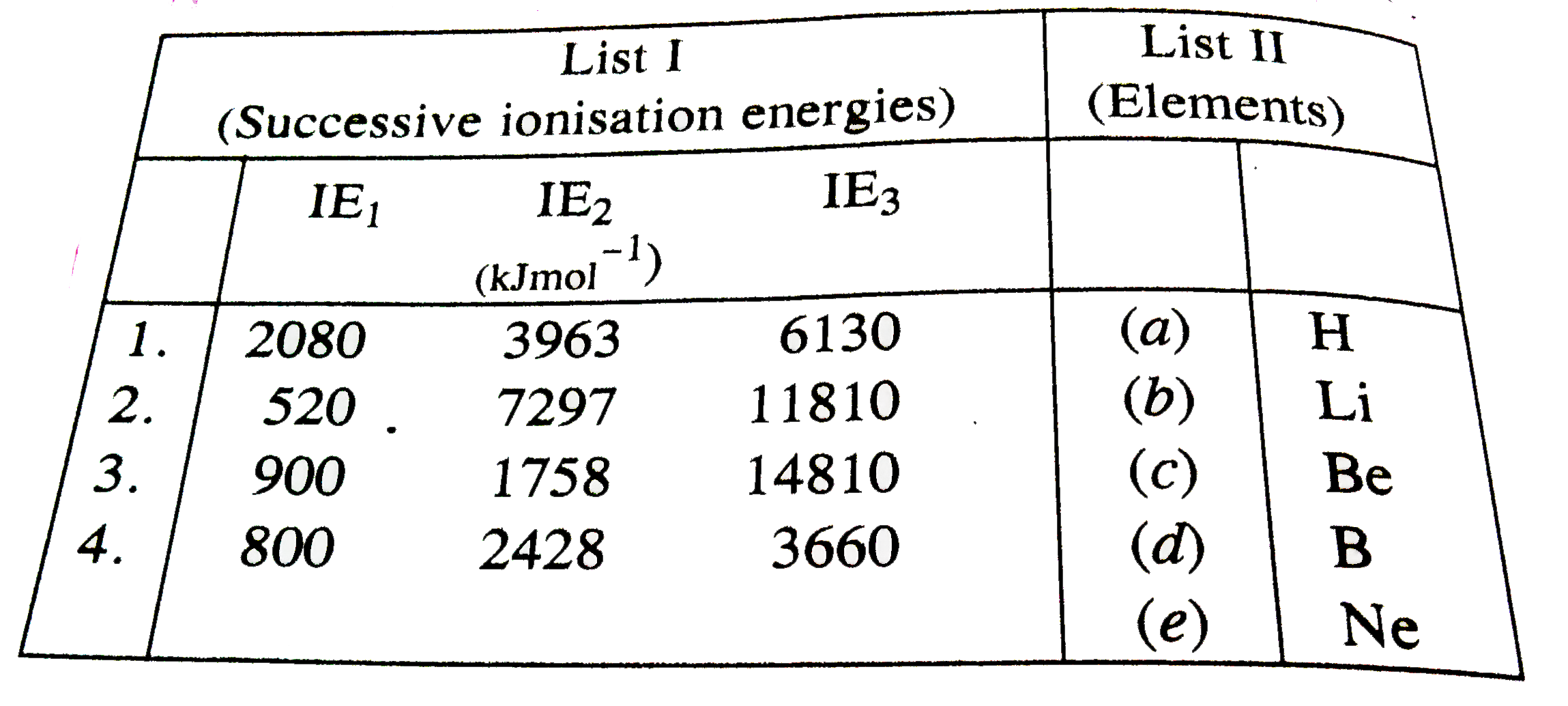A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES-All Questions
- Which of the following is an inert gas?
Text Solution
|
- Identify the correct order in which the covalent radius of the followi...
Text Solution
|
- Match 1list I with list II and select the correct answer using the cod...
Text Solution
|
- In the following, the element with the highest ionization energy is
Text Solution
|
- One mole of magnesium in the vapor state absored 1200 kJ mol^(-1) of e...
Text Solution
|
- Among the following transition elements, pick out the element/elements...
Text Solution
|
- Which of the following species has the highest electron affinity?
Text Solution
|
- The correct order of atomic sizes is
Text Solution
|
- Which electronic configuration of an element has abnormally high diffe...
Text Solution
|
- The electronic configurations of four elements are given below. Arrang...
Text Solution
|
- The electronic configuration of the atom having maximum difference in ...
Text Solution
|
- Which of the following two elements in the periodic table are expected...
Text Solution
|
- An element X belongs to fourth period and fifteenth group of the perio...
Text Solution
|
- The electronic configuration of the element with maximum electron affi...
Text Solution
|
- The first ionization energy of oxygen is less than that of nitrogen. W...
Text Solution
|
- The number of unpaired electrons in gaseous species of Mn^(3+), Cr^(3+...
Text Solution
|
- Which is the wrong order for the stated property ? .
Text Solution
|
- Lattice energy of an ionic compound depedns upon :
Text Solution
|
- Which one of the following order is correct for the first ionisation e...
Text Solution
|
- Correct sequence of increasing order of ionization energy is
Text Solution
|