A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATE OF MATTER (GASEOUS AND LIQUID STATE)
DINESH PUBLICATION|Exercise Assertion Reason|20 VideosS-BLOCK ELEMENTS (ALKALI AND ALKALINE EARTH METALS )
DINESH PUBLICATION|Exercise Assertion reason|12 VideosSTATES OF MATTER (SOLID STATE CHEMISTRY)
DINESH PUBLICATION|Exercise ULTIMATE PREPARATORY PACKAGE|21 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-STATE OF MATTER (GASEOUS AND LIQUID STATE)-Ulitmate Preparatory package
- If atomic mass of hydrogen on a hypothetical scale is choosen to be 10...
Text Solution
|
- Vapour density
Text Solution
|
- Unit of vapour density is
Text Solution
|
- The gas or vapour heavier than air is
Text Solution
|
- Under what conditions will a pure sample of an ideal gas not only exhi...
Text Solution
|
- The root mean square speed of the molecules of a diatomic gas is v. Wh...
Text Solution
|
- The R.M.S. Speed of the molecules of a gas of density kg m^(-3) and p...
Text Solution
|
- At a temperature T, K, the pressure of 4.0 gm argon in a bulb is P. Th...
Text Solution
|
- One mole each of a monoatomic, diatomic and triatomic gases are mixed ...
Text Solution
|
- One mole of an ideal monoatomic gas is mixed with 1 mole of an ideal d...
Text Solution
|
- A spark plug is not necessary in a diesel engine because
Text Solution
|
- CO(2) at 600 bar and a temperature above T(c) (T(c) = 304.15 K) is cal...
Text Solution
|
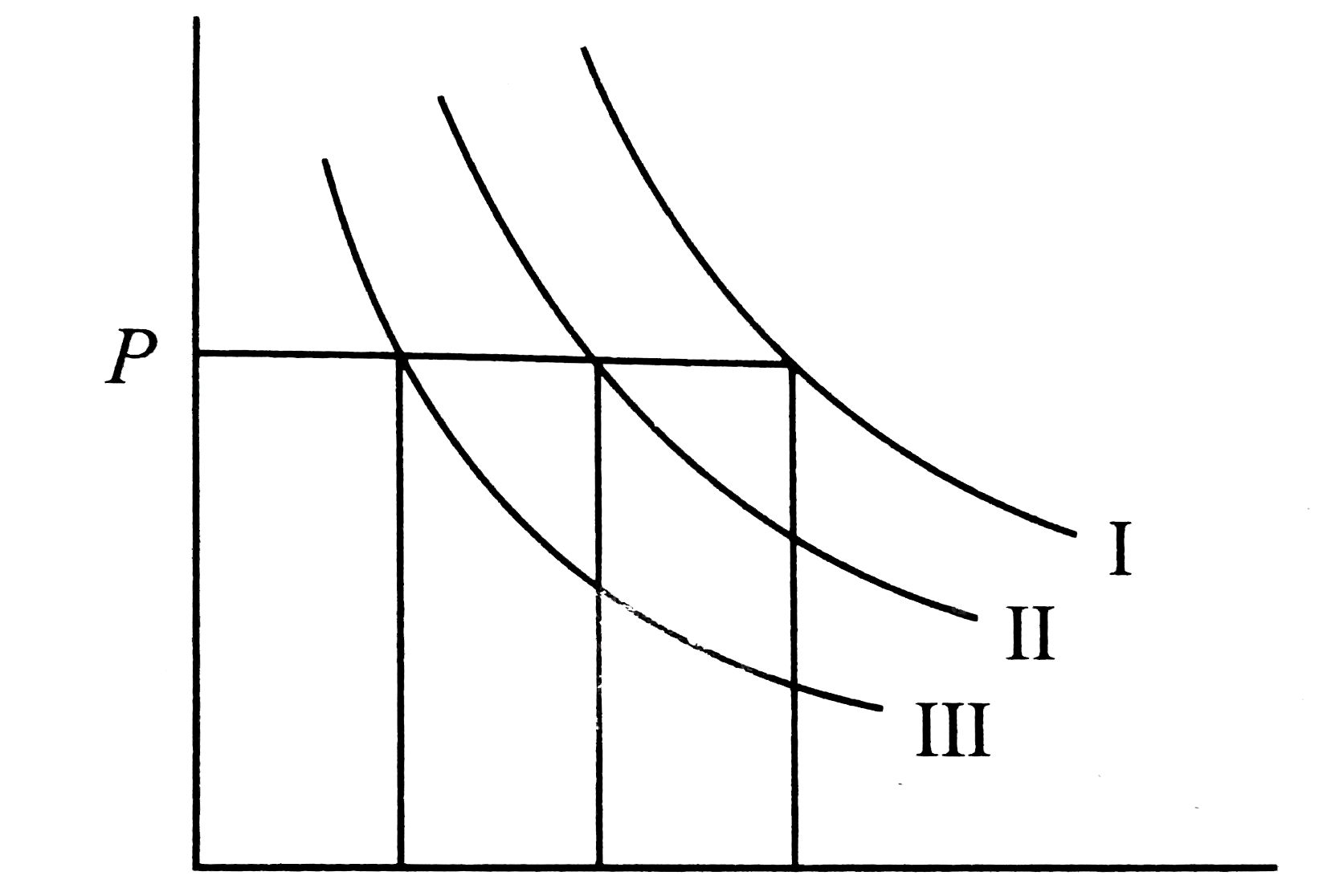
- I, II and III are three isotherms, respectively at T(1), T(2) and T(3)...
Text Solution
|
- Compressibility factor for H(2) behaving as real gas is:
Text Solution
|
- If bar(V) is the observed molor volume of real gas and bar(V)(id) is t...
Text Solution
|
- Vapour pressure of a pure liquid does not depend upon
Text Solution
|
- When super cooled freezes, its temperature suddenly rises, the Delta H...
Text Solution
|
- Two bubbles of different radii are connected by a hollow tube, then
Text Solution
|
- When 1 mole of super cooled water freezes, its temperature suddenly ri...
Text Solution
|
- Two identical fully insulated flasks X and Y contain the following F...
Text Solution
|