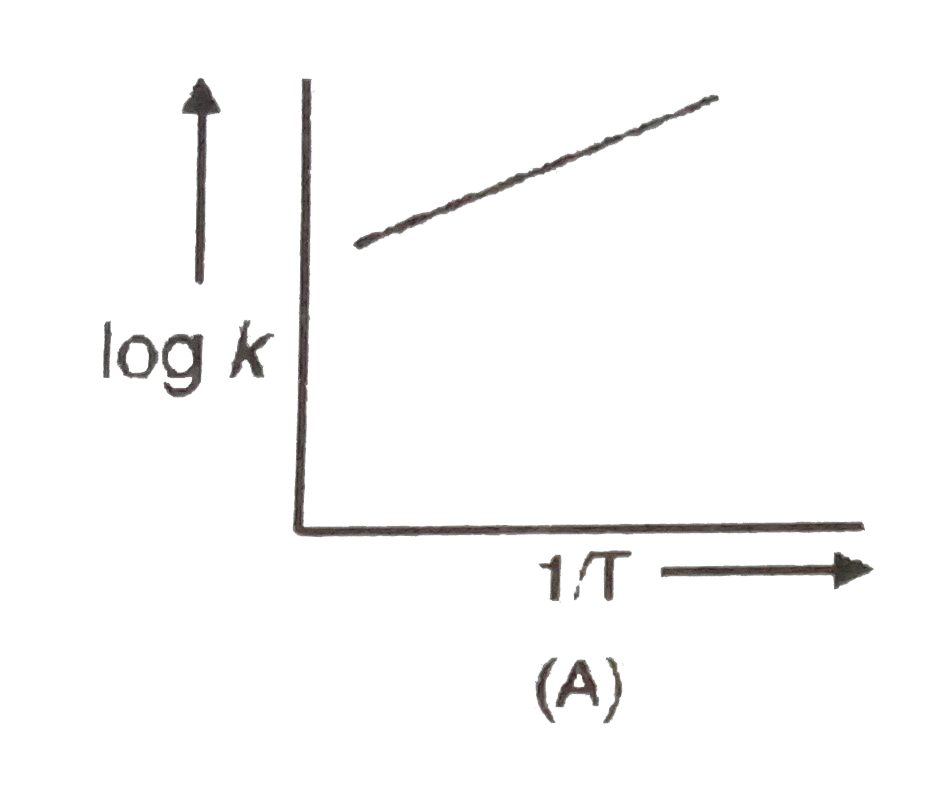
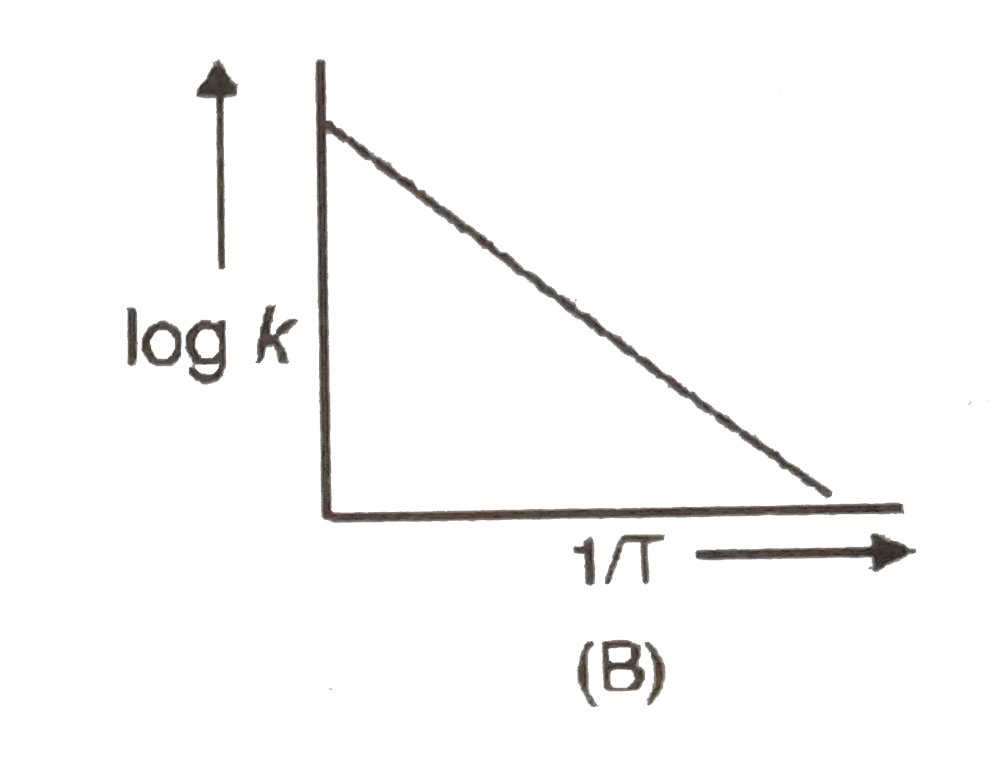
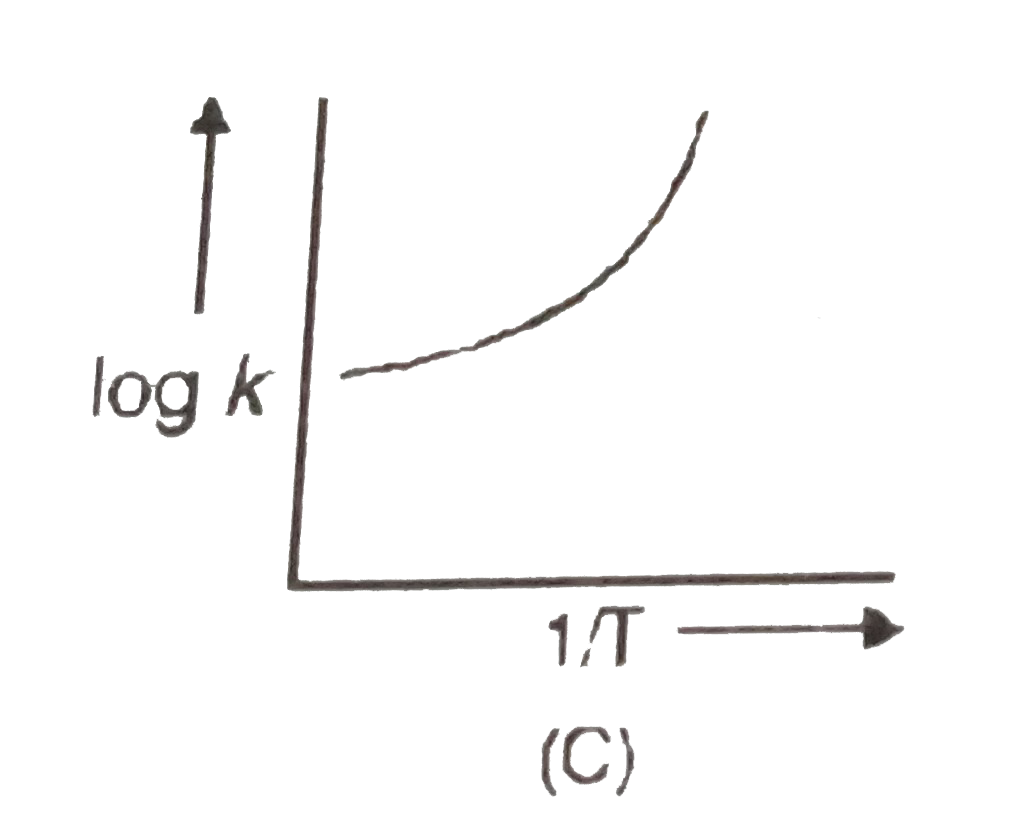
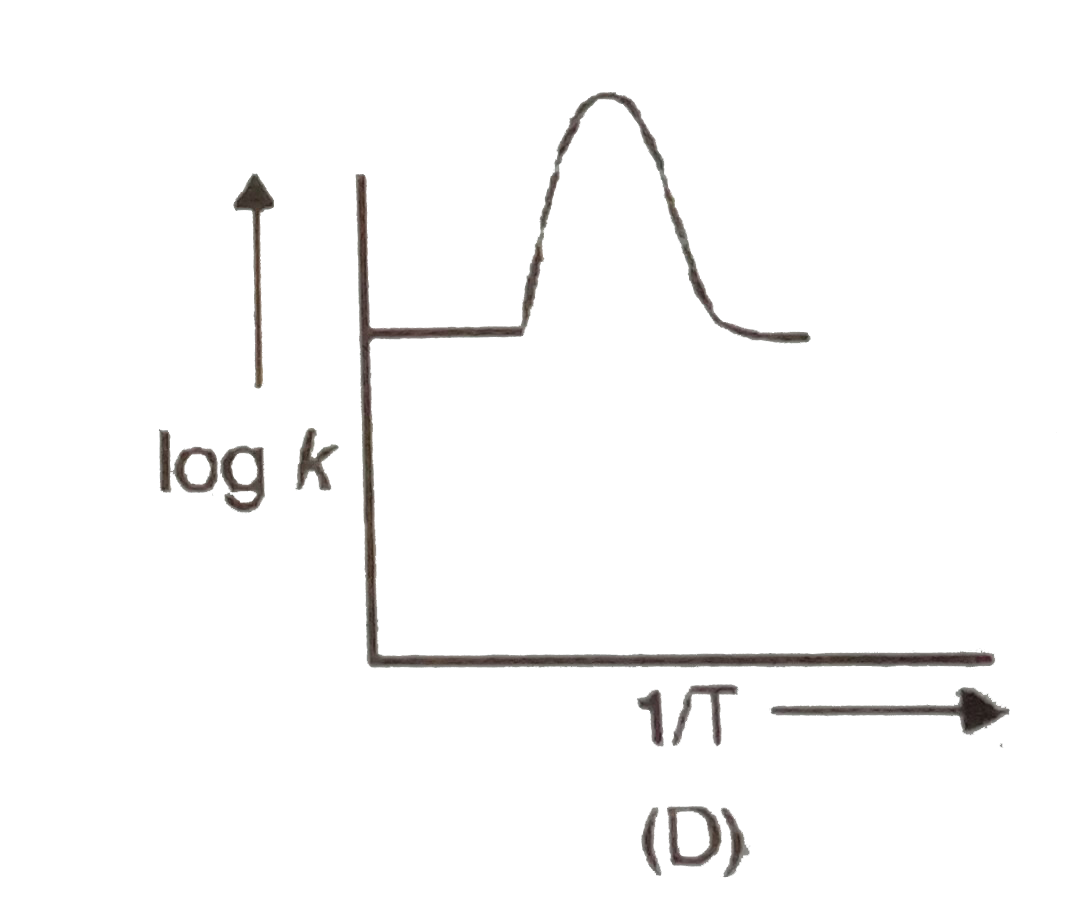
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
RATES OF REACTIONS AND CHEMICAL KINETICS
DINESH PUBLICATION|Exercise Selected Straight|49 VideosRATES OF REACTIONS AND CHEMICAL KINETICS
DINESH PUBLICATION|Exercise Comprehension|13 VideosRATES OF REACTIONS AND CHEMICAL KINETICS
DINESH PUBLICATION|Exercise Ultimate Preparatory Package|29 VideosPHYSICAL AND CHEMICAL EQUILIBRIA
DINESH PUBLICATION|Exercise ULTIMATE PREPARATORY PACKAGE|13 VideosREDOX REACTIONS
DINESH PUBLICATION|Exercise Reasoning Type Questions|4 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-RATES OF REACTIONS AND CHEMICAL KINETICS-Revision Question
- Half-life of a reaction is found to be inversely proportional to the c...
Text Solution
|
- The order of reaction is decided by
Text Solution
|
- A graph plotted between log k versus 1//T for calculating activation e...
Text Solution
|
- Units of rate constant of first and zero order reactions in terms of m...
Text Solution
|
- Which of the following is correct for a first order reaction ?
Text Solution
|
- 2A rarr B + C. It would be a zero-order reaction when
Text Solution
|
- If 3A rarr 2B, then the rate of reaction of + (dB)/(d t) is equal to
Text Solution
|
- When we increase the temperature, the rate of reaction increases becau...
Text Solution
|
- In Arrhenius plot, intercept is equal to
Text Solution
|
- The rate of reaction is doubled for 10^(@)C rise in temperature. The i...
Text Solution
|
- For the reaction: H(2) + Cl(2) underset("Sunlight")rarr2HCl taking...
Text Solution
|
- The temperature dependence of rate constant (k) of a chemical reaction...
Text Solution
|
- If the rate of the reaction is equal to the rate constant, the order o...
Text Solution
|
- The rate law for a reaction between the substances A and B is given by...
Text Solution
|
- Temperature coefficient of a reaction is 2. When temperature is increa...
Text Solution
|
- For the reaction A + Brarr C +D, if concentratiton of A is doubled wit...
Text Solution
|
- The potential energy diagram for a reaction R rarr P is given by ...
Text Solution
|
- Rate of 1st order depends upon
Text Solution
|
- For a first order reaction, units of rate constant are
Text Solution
|
- The unit of second order reaction rate constant is
Text Solution
|