A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
RATES OF REACTIONS AND CHEMICAL KINETICS
DINESH PUBLICATION|Exercise Selected Straight|49 VideosRATES OF REACTIONS AND CHEMICAL KINETICS
DINESH PUBLICATION|Exercise Comprehension|13 VideosRATES OF REACTIONS AND CHEMICAL KINETICS
DINESH PUBLICATION|Exercise Ultimate Preparatory Package|29 VideosPHYSICAL AND CHEMICAL EQUILIBRIA
DINESH PUBLICATION|Exercise ULTIMATE PREPARATORY PACKAGE|13 VideosREDOX REACTIONS
DINESH PUBLICATION|Exercise Reasoning Type Questions|4 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-RATES OF REACTIONS AND CHEMICAL KINETICS-Revision Question
- For the reaction : 2A +B rarr C +D, measurement of the rate of the rea...
Text Solution
|
- A first-order reaction was started with a decimolar solution of the re...
Text Solution
|
- A substance undergoes first order decomposition. The decomposition fol...
Text Solution
|
- For reaction aA rarr xP, when [A] = 2.2 mM, the rate was found to be 2...
Text Solution
|
- An endothermic reaction with high activation energy for the forward re...
Text Solution
|
- The energy of activations for forward and backward change for an endot...
Text Solution
|
- A reaction involiving two different reactants can never be:
Text Solution
|
- According to law of mass action rate of a chemical reaction is proport...
Text Solution
|
- For a first-order reaction A rarr B the reaction rate at reactant conc...
Text Solution
|
- The experimental rate law for a reaction 2A + 2B rarr Product, is ...
Text Solution
|
- What is the time required for a first order reaction to be 99 % comple...
Text Solution
|
- During the decomposition of H(2)O(2) to give oxygen, 48 g O(2) is form...
Text Solution
|
- If a homogeneous catalytic reaction can take place through three alter...
Text Solution
|
- Observe the following reaction 2A +B rarr C The rate of formation ...
Text Solution
|
- Half life of a radioactive substance is 6 minute. If its initial amoun...
Text Solution
|
- The rate law for a reaction between A and B is given by rate = k[A]^(n...
Text Solution
|
- Unit of the constant of zero order reaction is
Text Solution
|
- Arrhenius equation is
Text Solution
|
- t(1//4) can be taken as the time taken for concentration of reactant t...
Text Solution
|
- The rate of reaction between two A and B decreases by factor 4 if the ...
Text Solution
|
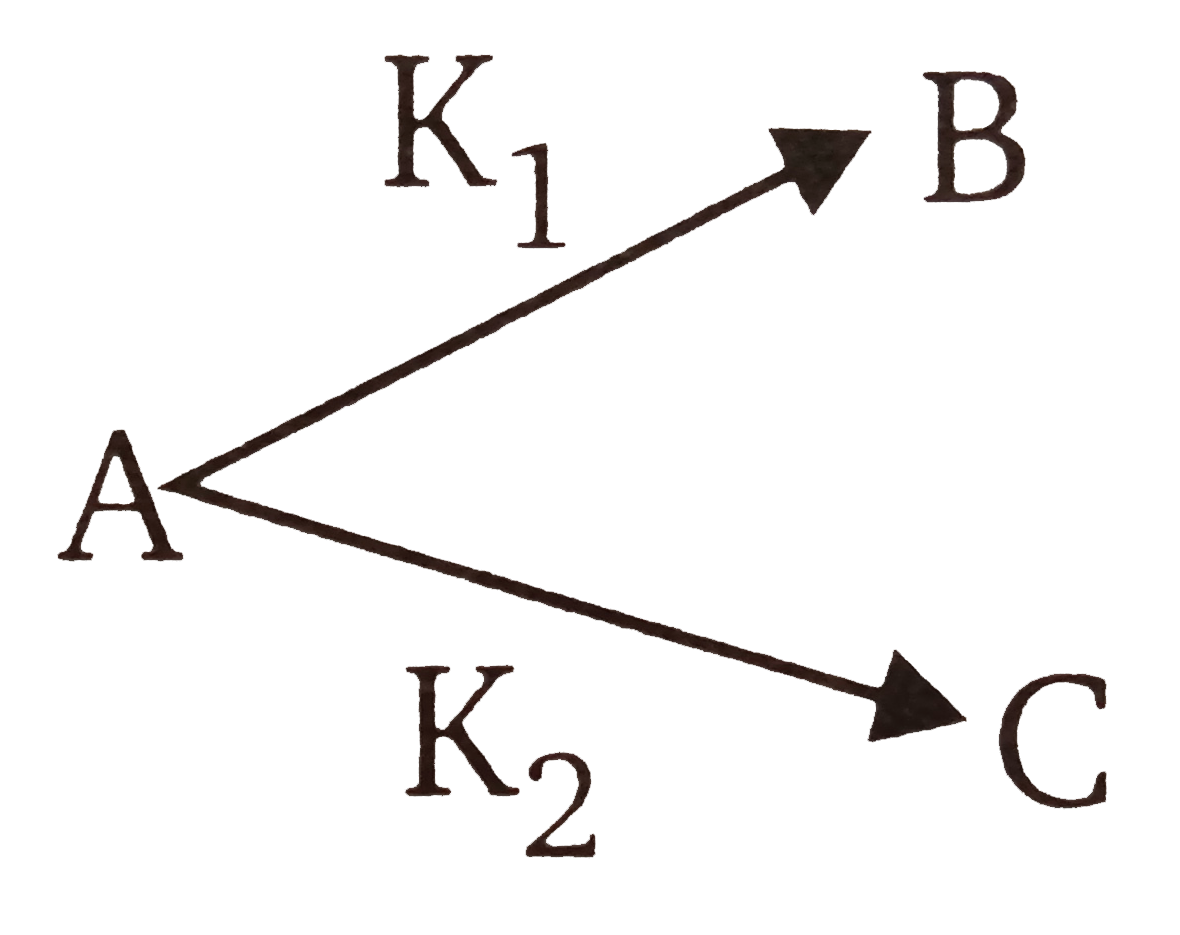 and `KP_(1)=1.26xx10^(-4)sec^(-1)`
and `KP_(1)=1.26xx10^(-4)sec^(-1)`