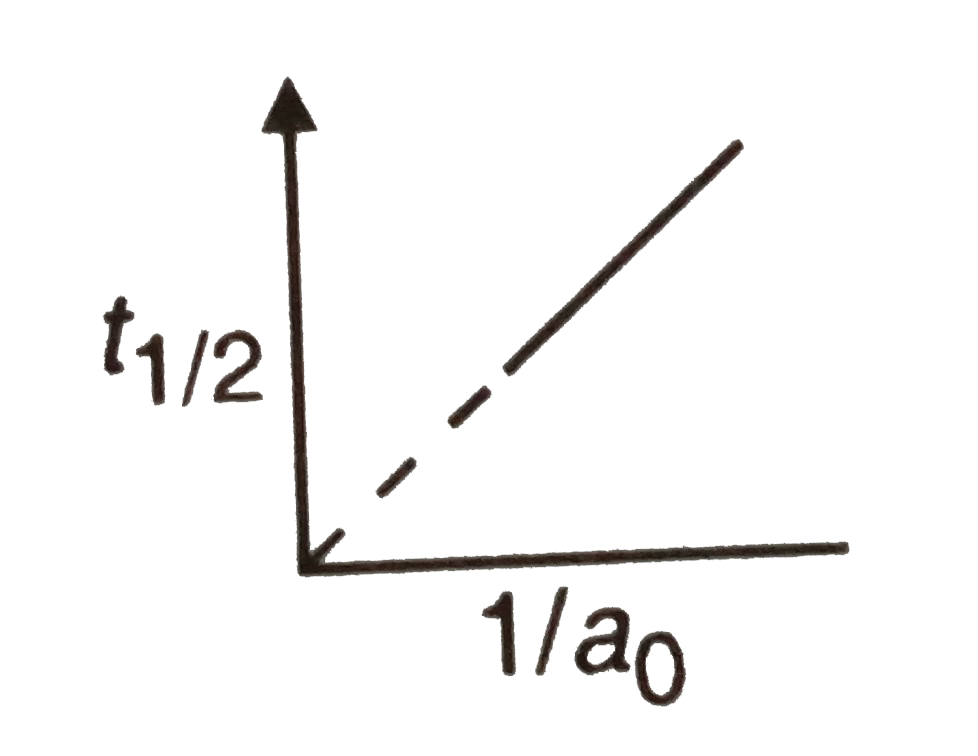A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
RATES OF REACTIONS AND CHEMICAL KINETICS
DINESH PUBLICATION|Exercise Selected Straight|49 VideosRATES OF REACTIONS AND CHEMICAL KINETICS
DINESH PUBLICATION|Exercise Comprehension|13 VideosRATES OF REACTIONS AND CHEMICAL KINETICS
DINESH PUBLICATION|Exercise Ultimate Preparatory Package|29 VideosPHYSICAL AND CHEMICAL EQUILIBRIA
DINESH PUBLICATION|Exercise ULTIMATE PREPARATORY PACKAGE|13 VideosREDOX REACTIONS
DINESH PUBLICATION|Exercise Reasoning Type Questions|4 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-RATES OF REACTIONS AND CHEMICAL KINETICS-Revision Question
- For a zero order reaction, the plot of concentration of a reactant vs ...
Text Solution
|
- The rate of reaction increases with temperature due to
Text Solution
|
- The following graph shows how t(1//2) (half-life) of a reactant R chan...
Text Solution
|
- For the reaction SO(2(g))+(1)/(2)O(2(g))hArrSO(3(g)), if K(c)=K(p)(RT)...
Text Solution
|
- The time taken for 10 % completion of a first order reaction is 20 min...
Text Solution
|
- The half life period of a radioactive substance is 10 hours. How much ...
Text Solution
|
- The rate law equation for a reaction A rarr B is , r = k[A]^(0) If...
Text Solution
|
- For a chemical reaction Ararr B,the rate of the reaction is 2.0xx10^(-...
Text Solution
|
- For the decomposition of a compount AB at 600 K, the following data we...
Text Solution
|
- For a reaction between A and B, the initial rate of reaction is measur...
Text Solution
|
- For the first order reaction at 27^(@)C, the ratio of time required fo...
Text Solution
|
- For a first order reaction the rate constant is 6.909 "min"^(-1). The ...
Text Solution
|
- The unit "mol L"^(-1)s^(-1) is meant for the rate constant of the reac...
Text Solution
|
- The half life period of a reaction is inversely proportional to the sq...
Text Solution
|
- For a first order reaction A rarr Products, the half life is 100 secon...
Text Solution
|
- The rate of a gaseous reaction triples when temperature is increased b...
Text Solution
|
- The reaction N(2)O(5) (in C Cl(4) solution) rarr 2NO(2) (solution) +...
Text Solution
|
- The first order reaction 2N(2)O(g)rarr2N(2)(g)+O(2)(g) has a rate cons...
Text Solution
|
- In Arrhenius equation for activation energy, k=Ae^(-E(a)//RT), A repre...
Text Solution
|
- In the reaction A +2B rarr C +2O the initial rate (-d[A])/(dt) at t=0w...
Text Solution
|