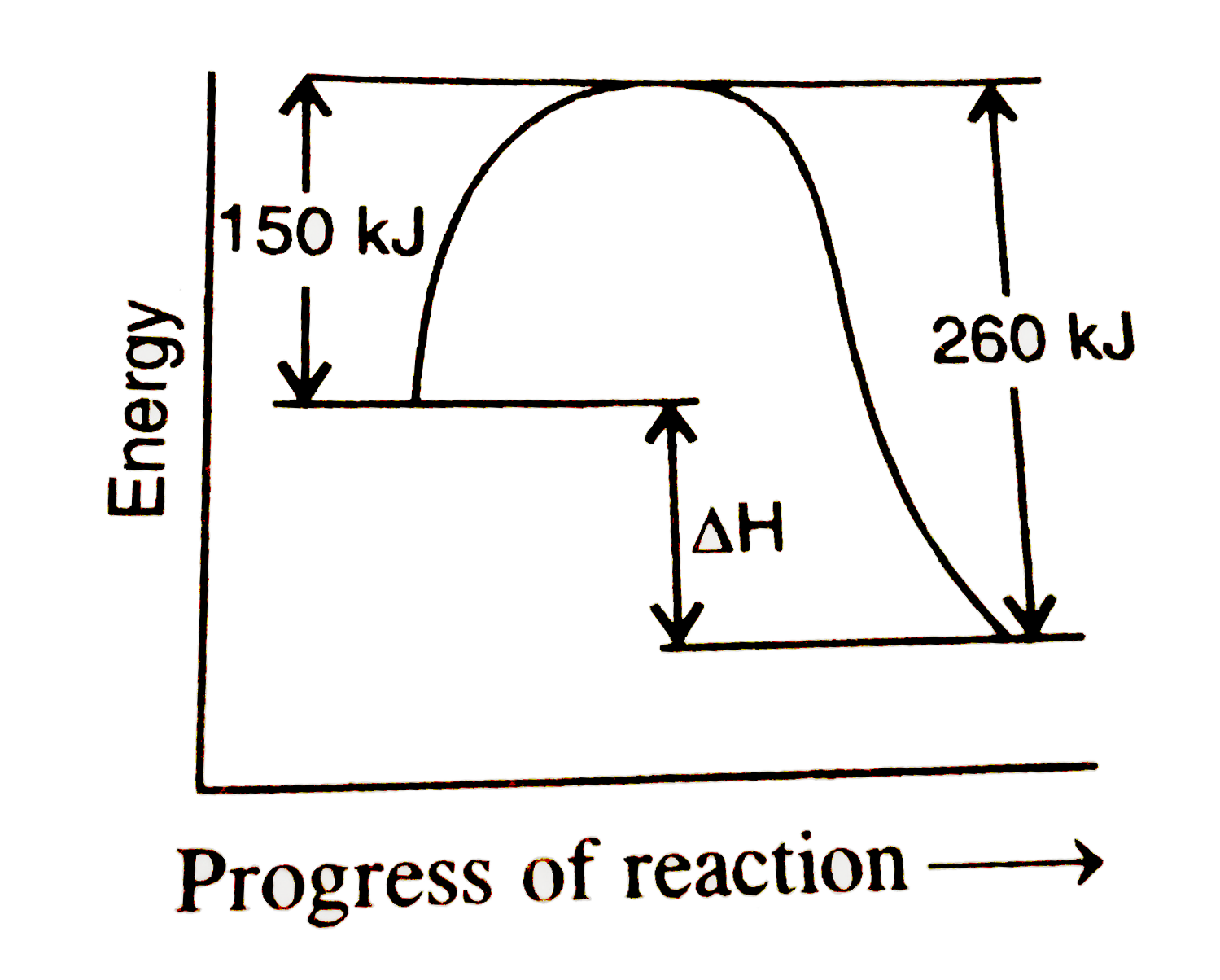
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
RATES OF REACTIONS AND CHEMICAL KINETICS
DINESH PUBLICATION|Exercise Selected Straight|49 VideosRATES OF REACTIONS AND CHEMICAL KINETICS
DINESH PUBLICATION|Exercise Comprehension|13 VideosRATES OF REACTIONS AND CHEMICAL KINETICS
DINESH PUBLICATION|Exercise Ultimate Preparatory Package|29 VideosPHYSICAL AND CHEMICAL EQUILIBRIA
DINESH PUBLICATION|Exercise ULTIMATE PREPARATORY PACKAGE|13 VideosREDOX REACTIONS
DINESH PUBLICATION|Exercise Reasoning Type Questions|4 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-RATES OF REACTIONS AND CHEMICAL KINETICS-Revision Question
- In the reaction A +2B rarr C +2O the initial rate (-d[A])/(dt) at t=0w...
Text Solution
|
- Half life of a first order reaction and a zero order reaction are same...
Text Solution
|
- If the activation enery for the forward reaction is 150 "kJ mol"^(-1) ...
Text Solution
|
- The rate law for the reaction 2X +Y rarr Z is Rate = k[X][Y] The c...
Text Solution
|
- Consider the following statements (i) increase in the concentration ...
Text Solution
|
- The activation energy for a reaction at temperature T K was found to b...
Text Solution
|
- The time required for 100% completion of a zero order reaction is
Text Solution
|
- The following data is obtained during the first order thermal decompos...
Text Solution
|
- Consider the decomposition of N(2)O(5) as N(2)O(5)rarr2NO(2)+1//2O(2...
Text Solution
|
- For the reaction R rarr P, graph of [R] against time is found to be a ...
Text Solution
|
- In a catalyst experiment involving the Haber process N(2) + 3H(2) rarr...
Text Solution
|
- 10 g of a radioactive isotope is reduced to 1.25 g in 12 years. Theref...
Text Solution
|
- Plots showing the variation of the rate constant (k) with temperature ...
Text Solution
|
- The rate of the reaction A rarr products, at the initial concentration...
Text Solution
|
- For first order reaction, the time taken to reduce tha initial concent...
Text Solution
|
- The initial rates of reaction 3 A+ 2B +C rarr products at different in...
Text Solution
|
- The activation energy for a reaction at temperature T K was found to b...
Text Solution
|
- Units of rate constant depend upon
Text Solution
|
- Which one of the following statements for the order of a reaction is i...
Text Solution
|
- The rate of law for the reaction xA+yB=mP+nQ is Rate k[A]^(c)[B]^(d). ...
Text Solution
|