A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
RATES OF REACTIONS AND CHEMICAL KINETICS
DINESH PUBLICATION|Exercise Comprehension|13 VideosRATES OF REACTIONS AND CHEMICAL KINETICS
DINESH PUBLICATION|Exercise Matrix Match|2 VideosRATES OF REACTIONS AND CHEMICAL KINETICS
DINESH PUBLICATION|Exercise Revision Question|168 VideosPHYSICAL AND CHEMICAL EQUILIBRIA
DINESH PUBLICATION|Exercise ULTIMATE PREPARATORY PACKAGE|13 VideosREDOX REACTIONS
DINESH PUBLICATION|Exercise Reasoning Type Questions|4 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-RATES OF REACTIONS AND CHEMICAL KINETICS-Selected Straight
- The rate constant of a reaction is given by k = 2.1 xx 10^(10) exp(-27...
Text Solution
|
- Arrhenius equation may be represented as
Text Solution
|
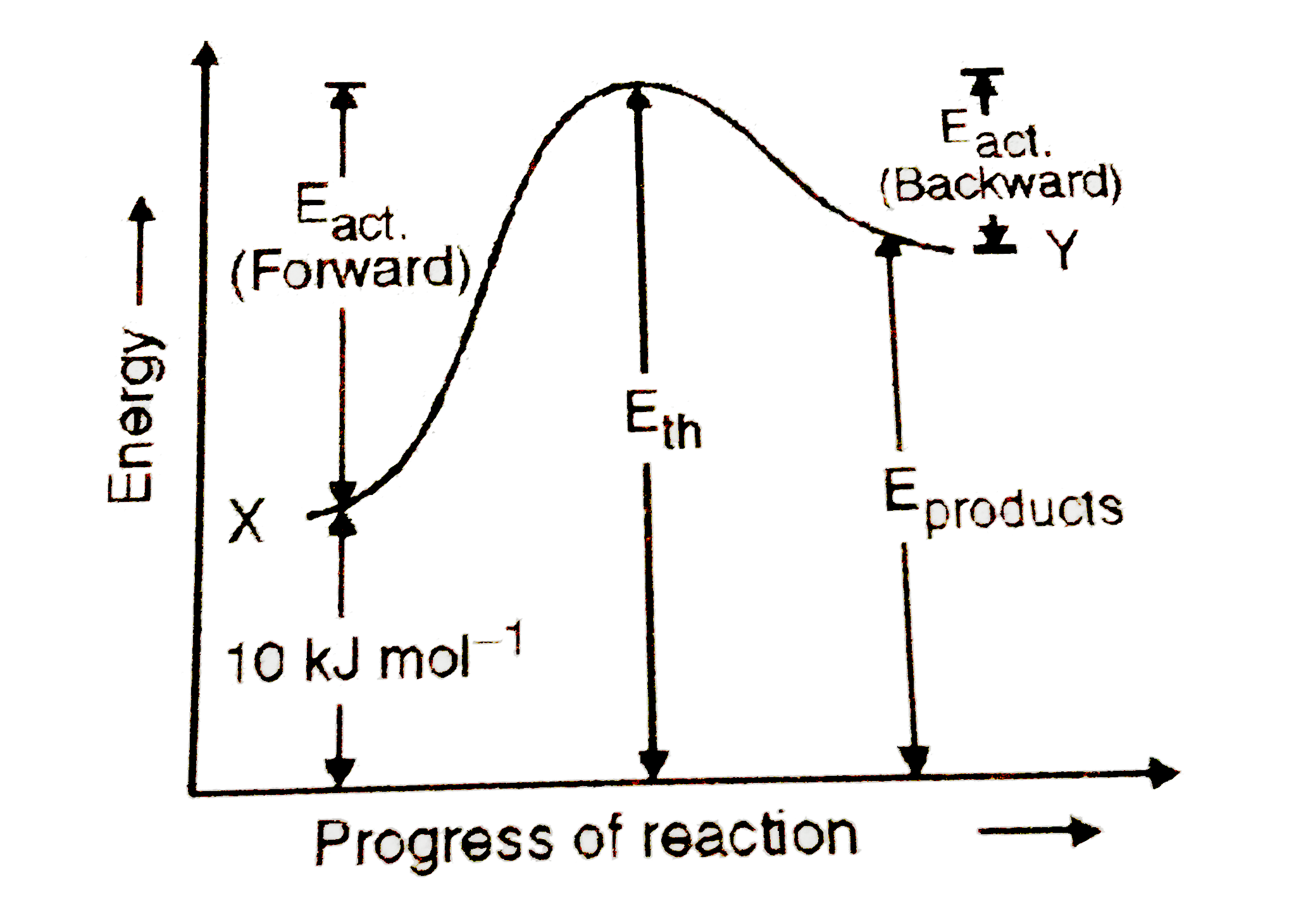
- In a pypothetical reaction X rarr Y , the activation are 15 and 9 "kJ ...
Text Solution
|
- Taking the reaction, A +2B rarr Products to be of second order, which...
Text Solution
|
- Which of the following correctly represent/s the units of rate of reac...
Text Solution
|
- Point out the correct statement/s
Text Solution
|
- A catalyst
Text Solution
|
- For a first order reaction,
Text Solution
|
- The following statement (s) is/are correct
Text Solution
|
- The specific rate constant of a first order reaction depends on the
Text Solution
|
- A catalyst is a substance which
Text Solution
|
- A catalyst
Text Solution
|
- The rate law for the reaction RCl + NaOH(aq) rarr ROH + NaCl is give...
Text Solution
|
- The temperature coefficient of most of the reactions lies between
Text Solution
|
- For an endothermic reaction, where Delta H represents the enthalpy of ...
Text Solution
|
- The rate constant, the activation energy, and the Arrhenius parameter ...
Text Solution
|
- The rate constant for the reaction: 2N(2)O(5) rarr 4NO(2)+O(2) is 3....
Text Solution
|
- When two reactants, A and B are mixed to give products C and D, the re...
Text Solution
|
- If I is the intenisty of an absorbed light and c is the concentration ...
Text Solution
|
- Conisder the chemical reaction N(2)(g) + 3H(2)(g) rarr 2NH(3)(g) T...
Text Solution
|