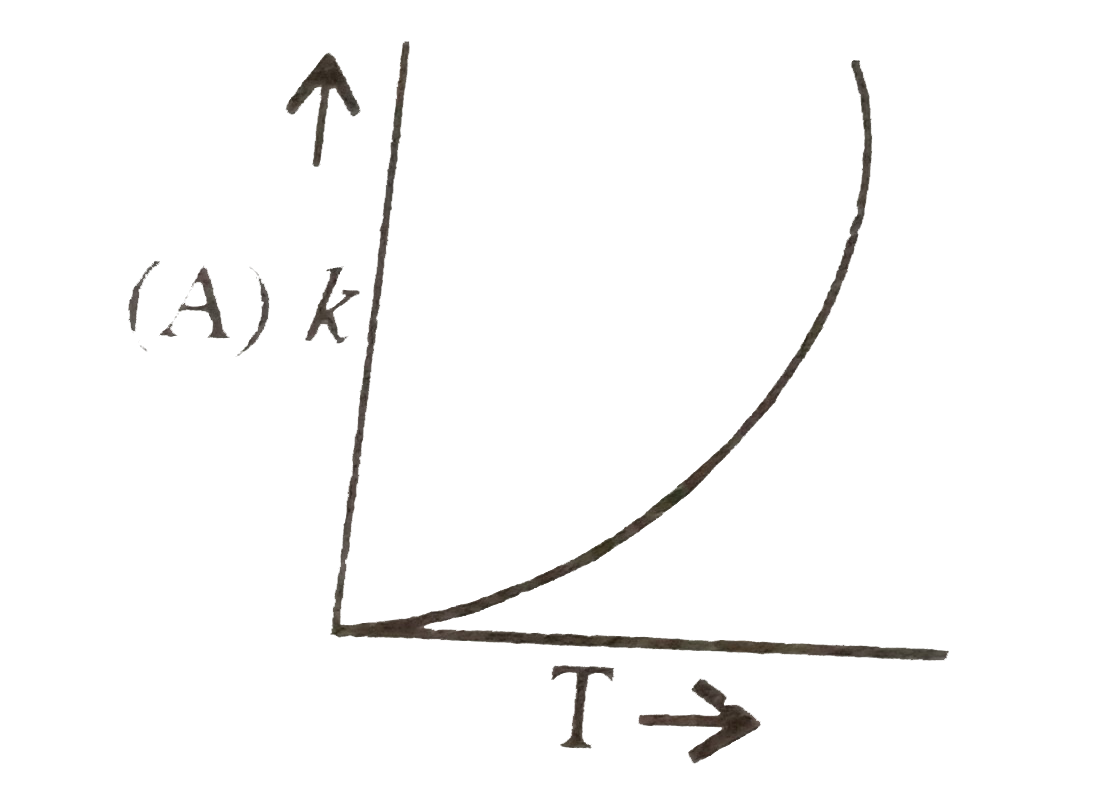
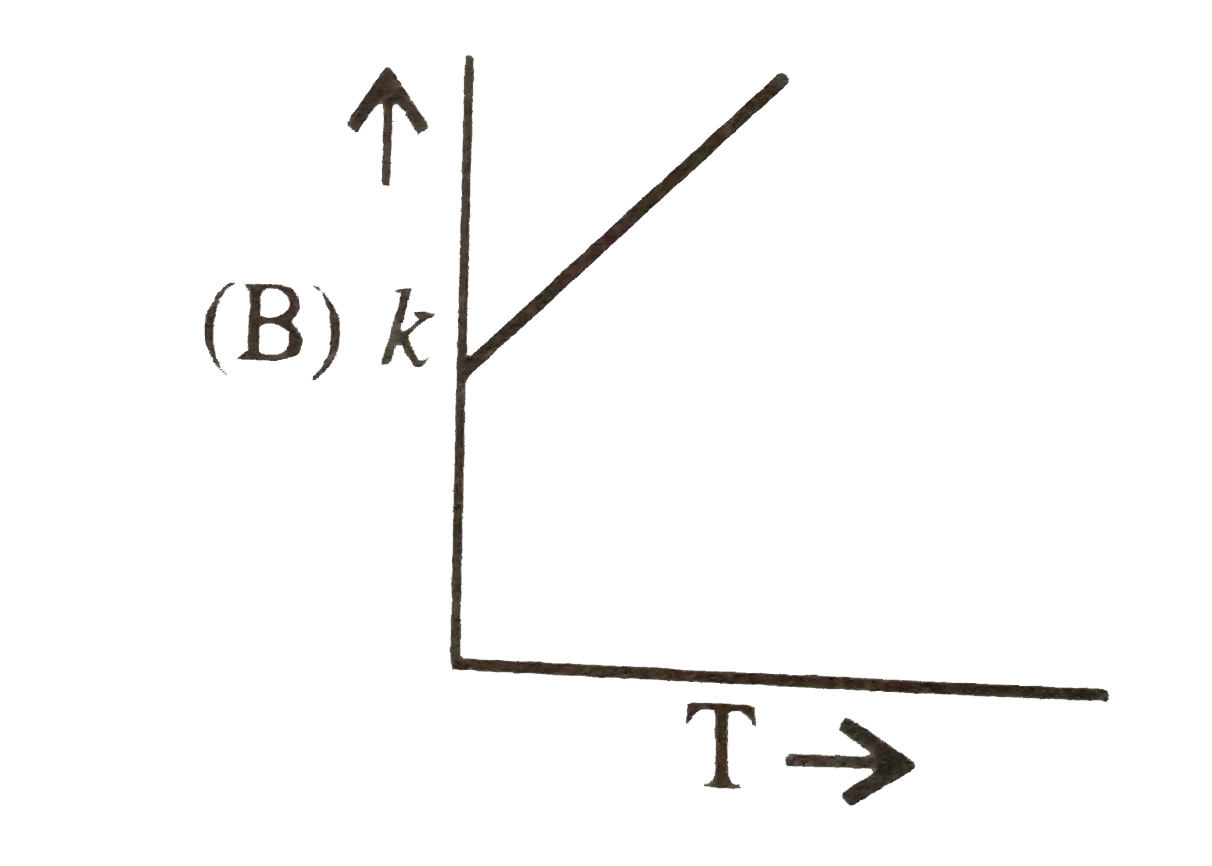
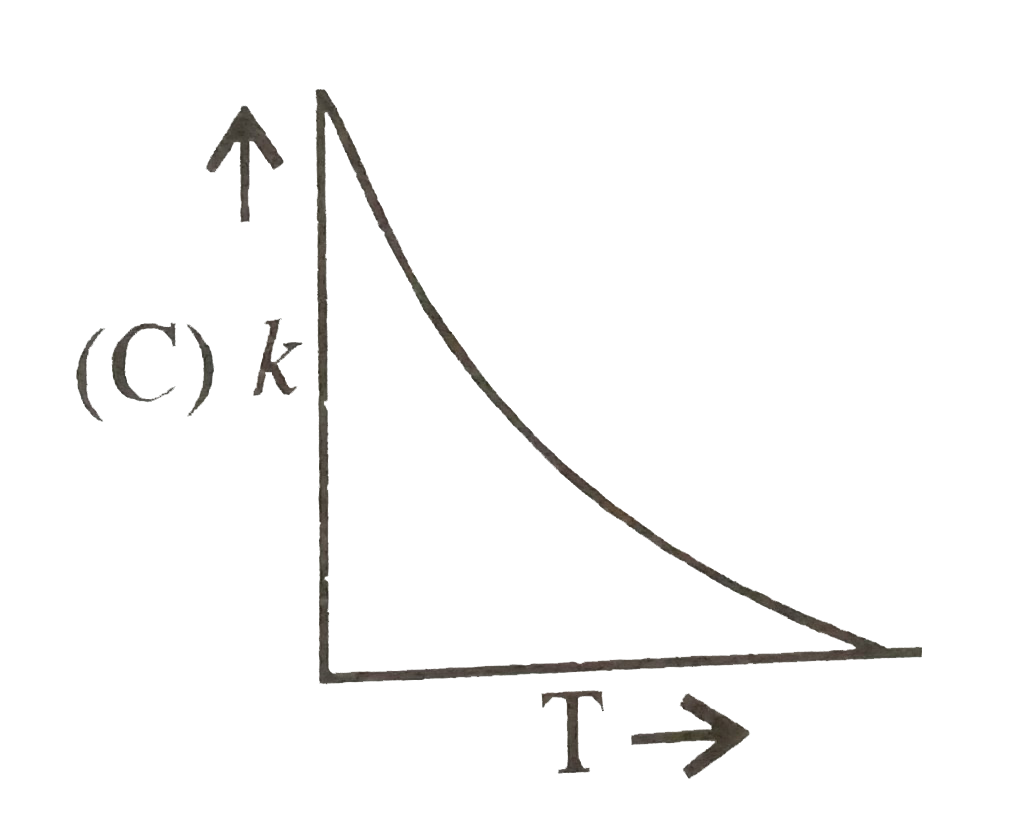
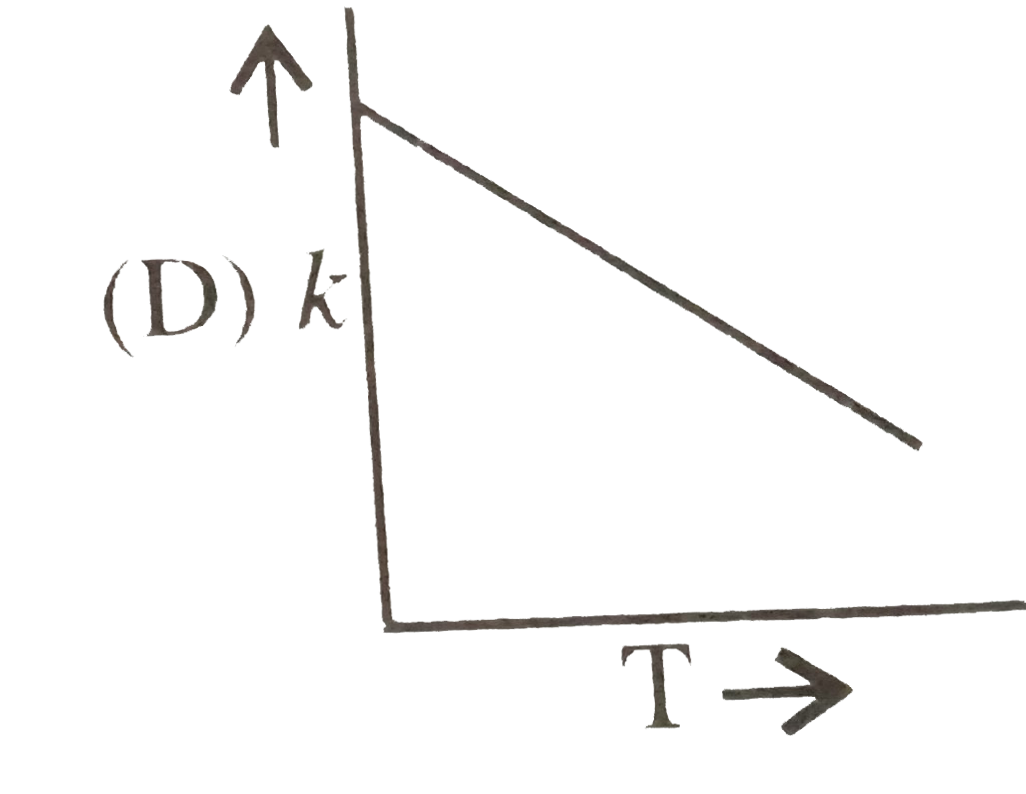
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
RATES OF REACTIONS AND CHEMICAL KINETICS
DINESH PUBLICATION|Exercise Comprehension|13 VideosRATES OF REACTIONS AND CHEMICAL KINETICS
DINESH PUBLICATION|Exercise Matrix Match|2 VideosRATES OF REACTIONS AND CHEMICAL KINETICS
DINESH PUBLICATION|Exercise Revision Question|168 VideosPHYSICAL AND CHEMICAL EQUILIBRIA
DINESH PUBLICATION|Exercise ULTIMATE PREPARATORY PACKAGE|13 VideosREDOX REACTIONS
DINESH PUBLICATION|Exercise Reasoning Type Questions|4 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-RATES OF REACTIONS AND CHEMICAL KINETICS-Selected Straight
- Which of the following is incorrect about the order of reaction ?
Text Solution
|
- Rate of a reaction can be expressed by Arrhenius equation as: k = Ae...
Text Solution
|
- The following mechanism has been proposed for the reaction of NO with ...
Text Solution
|
- In a first-order reaction A rarr B, if K is the rate constant and init...
Text Solution
|
- The reaction obey I order with respect to H(2) and ICl both. H(2) (g...
Text Solution
|
- If 60 % of a first order reaction was completed in 60 minutes, 50 % of...
Text Solution
|
- The energies of activation for forward and reverse reaction for A(2)+...
Text Solution
|
- Consider a reaction, 2A + B rarr Products When concentration of B al...
Text Solution
|
- Conisder a reaction aG+bH rarr Products. When concentration of both th...
Text Solution
|
- Under the same reaction conditions, the intial concentration of 1.386 ...
Text Solution
|
- For the reaction A + B products, it is observed that: (1) on doublin...
Text Solution
|
- Half-life period of a first-order reaction is 1386 seconds. The specif...
Text Solution
|
- The half-life period of a first-order chemical reaction is 6.93 min. T...
Text Solution
|
- The time for half-life period of a certain reaction, A rarr products i...
Text Solution
|
- Consider the reaction, Cl(2)(aq) + H(2)S(aq) rarr S(s) + 2H^(+) (aq)...
Text Solution
|
- For the reaction N(2)O(5) rarr 2NO(2) + (1)/(2) O(2), the rate of disa...
Text Solution
|
- During the kinetic study of the reaction 2A +B rarr C + D following ...
Text Solution
|
- Plots showing the variation of the rate constant (k) with temperature ...
Text Solution
|
- The rate of reaction: 2NO + Cl(2) rarr 2NOCl is given by the rate, e...
Text Solution
|
- The rate of a chemical reaction doubles for every 10^(@)C rise of temp...
Text Solution
|