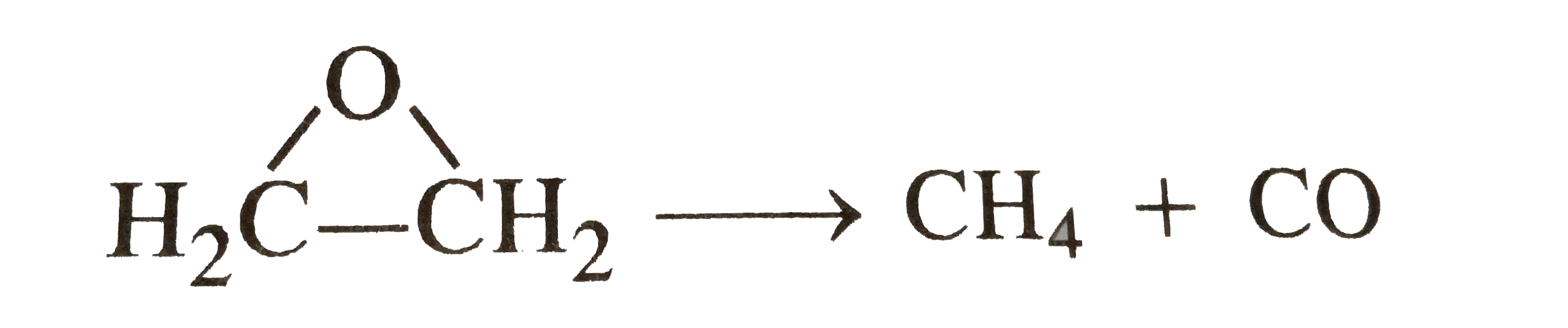A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-RATES OF REACTIONS AND CHEMICAL KINETICS-Ultimate Preparatory Package
- A certain reaction A+B rarr C is first order with respect to each reac...
Text Solution
|
- The decomposition of N(2)O into N(2) and O in the presence of gaseous ...
Text Solution
|
- The rate constant for the first order decomposition of ethylene oxide ...
Text Solution
|
- A substance X decomposes in solution following the first order kinetic...
Text Solution
|
- In a second order reaction, first order in each reactant A and B, whic...
Text Solution
|
- For a first order reaction, the ratio of time for the completion of 99...
Text Solution
|
- For the reaction R rarr P when concentration of R is made double the r...
Text Solution
|
- The chemical reaction 2O(3) overset(k(1))rarr 3O(2) proceeds as follow...
Text Solution
|
- The concentration of a reactant in solution falls from (i) 0.5 M to 0....
Text Solution
|
- In a particular reaction t(1//2) was found to increase 16 times when i...
Text Solution
|
- For a zero order reaction a graph of conc. (along Y axis) and time (al...
Text Solution
|
- The rate constant of a zero order reaction is 0.2 mol dm^(-3) "min"^(-...
Text Solution
|
- For a hypothetical reaction R rarr P the rate constant is 2944.06 s^(-...
Text Solution
|
- For a zero order reaction a plot of rate (along Y-axis) and concentra...
Text Solution
|
- At 373 k, the following reaction A(g)rarr2B(g)+C(g) is found to be of...
Text Solution
|
- A reaction has a rate constant of 0.25 "mol"^(-1) L s^(-1) if the init...
Text Solution
|
- In a reversible reaction the rate of the backward reaction is
Text Solution
|
- Hydrolysis of amyl acetate was carried out separately in the presence ...
Text Solution
|
- When number of reactants are very large the method used to find the or...
Text Solution
|
- Which of the following is not used to find the order of reaction ?
Text Solution
|