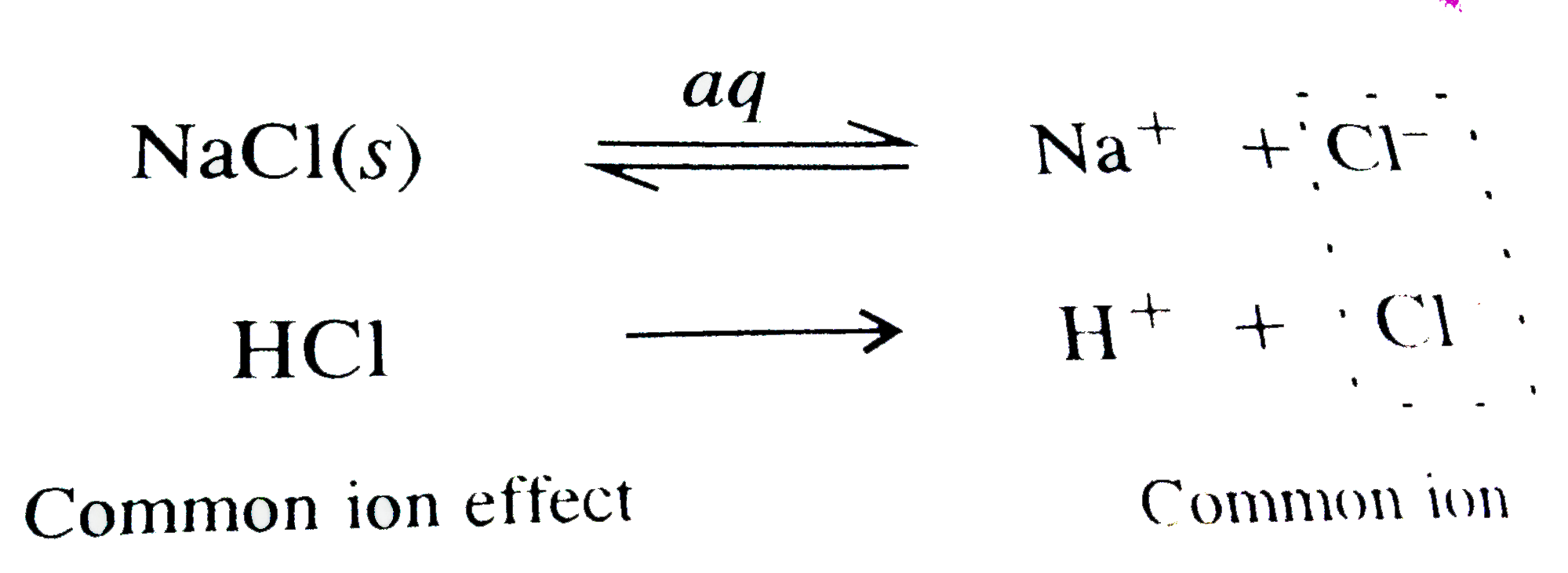A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-THE ALKALI METALS-ULTIMATE PREPARATORY PACKAGE
- Pure sodium chloride is prepared by saturating a cold saturated soluti...
Text Solution
|
- Which among the following is most soluble in alcohol ?
Text Solution
|
- Which of the following is used as a source of O(2) in space capsules, ...
Text Solution
|
- Li(2)O(2) is formed when
Text Solution
|
- Most insoluble salt of Li in water is
Text Solution
|
- The order of solubility of lithium halides in non-lolar solvents follo...
Text Solution
|
- Melting points of chlorides of alkali metals follow the order
Text Solution
|
- Which of the following is not correct ?
Text Solution
|
- Rb[ICI(2)] on heating gives
Text Solution
|
- Which of the following is white ?
Text Solution
|
- Which of the following is not a white crystalline solid ?
Text Solution
|
- The most insoluble salt of sodium is
Text Solution
|
- A colloidal solution of Na in ether is
Text Solution
|
- Pick the odd one cut
Text Solution
|
- Pick the odd one out
Text Solution
|
- The alkali metal which can be obtained by reduction of its carbonate w...
Text Solution
|
- The alkali metal which can be obtained by metal displacement is
Text Solution
|
- A highly concentrated solution (about 5 M) of an alkali metal in ammon...
Text Solution
|
- A moderately concentrated solution of an alkali metal in NH(3) (pick t...
Text Solution
|
- Pick the odd one out
Text Solution
|