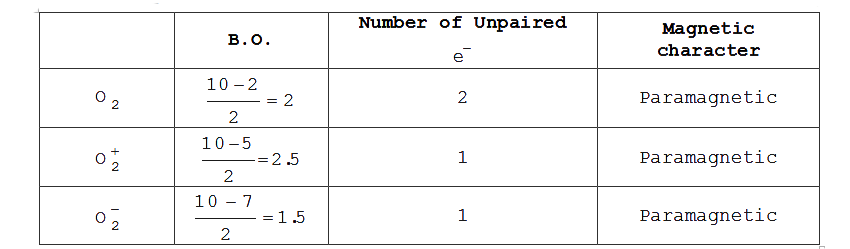Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Using molelcular orbital theory, compare the bond energy and magnetic ...
Text Solution
|
- Using molecular orbital theory, compare the bond energy and magnetic c...
Text Solution
|
- With the help of molecular orbital theory predict which of the followi...
Text Solution
|
- Using molelcular orbital theory, compare the bond energy and magnetic ...
Text Solution
|
- Compare the relative stability of the following species and indicate t...
Text Solution
|
- According to molecular orbital theory, which of the following statemen...
Text Solution
|
- Using molecular orbital theory predict the correct decreasing bond ord...
Text Solution
|
- Compare the relative stability of the following species and indicate t...
Text Solution
|
- Compare the relative stability of the following species and indicate t...
Text Solution
|