Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
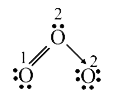
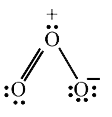
- The formal charges on the three O atoms in the O3 molecule are
Text Solution
|
- The formal charge of the O-atom in the ion [: ddotN = O:] is
Text Solution
|
- The formal charges on the three O atoms in the O3 molecule are
Text Solution
|
- The formal charge on P atom in orthophosphoric acid molecule is
Text Solution
|
- Calculate formal charge on each O-atom of O(3) molecule.
Text Solution
|
- The formal charges on the three O atoms in the O3 molecule are
Text Solution
|
- In O(3) molecule, the formal charge on the central O-atom is-
Text Solution
|
- The formal charges on the three oxygen atoms in O(3) molecule are
Text Solution
|
- Formal charge on two O atoms in
Text Solution
|