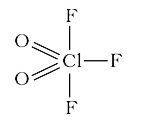
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The hybridization of the central atom in ClO(2)F(3) is :
Text Solution
|
- The type of hybrid orbitals used by chlorine atom in ClO(2)^(-) is :
Text Solution
|
- The type of hybrid orbitals used by chlorine atom in ClO(3)^(-) is
Text Solution
|
- NO(3)^(-) आयन के केन्द्रीय नाइट्रोजन परमाणु पर संकरण है :
Text Solution
|
- X(e)F(2) तथा XeF(4) में उपस्थित केन्द्रीय परमाणु में संकरण का केवल प्र...
Text Solution
|
- किस अणु के केन्द्रीय परमाणु में sp^(3) संकरण है ?
Text Solution
|
- What is the geometry and hybridisation of the central atom of the foll...
Text Solution
|
- The hybridization of the central atom in ClO(2)F(3) is :
Text Solution
|
- Among the following molecules how many are having sp^(3)d hybridizatio...
Text Solution
|