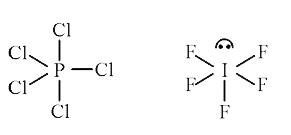
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Explain why PCl(5) is trigonal bipyramidal whereas IF(5) is square pyr...
Text Solution
|
- Explain why PCl(5) is trigonal bipyramidal whereas IF(5) is square pyr...
Text Solution
|
- Statement-1 : PCl(5) has trigonal bipyramidal shape . and Statement-2 ...
Text Solution
|
- Explain why PCl(5) is trigonal bipyramidal whereas IF(5) is square pyr...
Text Solution
|
- Match the entries of column I with appropriate entries of coloumn II a...
Text Solution
|
- स्पष्ट कीजिए कि PCl(5) त्रिकोणीय द्वीपीरामिडीय तथाIF(5) वर्ग पिरामिडी...
Text Solution
|
- Among the following statements:- I. PCl(5) is trigonal bipyramidal whe...
Text Solution
|
- Observe the following molecules : PCl(5) , BrF(5), ClF(5), PF(5)ClF(3)...
Text Solution
|
- The shape of SF(6) molecule is octahedral whereas that of IF(7) is squ...
Text Solution
|