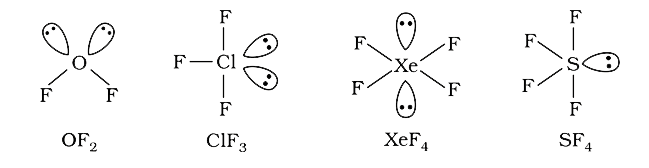A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which among the following is (are) having two lone pair of electrons o...
Text Solution
|
- Which among the following is (are) having two lone pair of electrons o...
Text Solution
|
- In which , central atom s has have one lone pair of electron ?
Text Solution
|
- In which of the following, the central atoms has two lone pairs of ele...
Text Solution
|
- In which of the following , central atom does not have one lone pairs ...
Text Solution
|
- In which of the following the central atom has two lone pairs of elect...
Text Solution
|
- The compound with two lone pairs of electrons on the central atom is (...
Text Solution
|
- Which of the following pair contains 2 long pair of electrons on the c...
Text Solution
|
- Which of the following pair contains 2 lone pairs of electrons on the ...
Text Solution
|