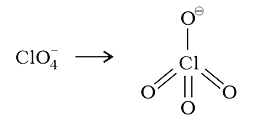
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider the following molecules or ions CH2Cl2 (ii) NH4^+ (iii) SO...
Text Solution
|
- In which of the following molecules/ions in the central atom sp^2-hybr...
Text Solution
|
- Only sp and sp^(2) hybrid orbitals are involved in the formation of :
Text Solution
|
- Consider the following molecules or ions CH2Cl2 (ii) NH4^+ (iii) SO4^(...
Text Solution
|
- Assertion(A) - NO3^- ion has same geometry as NH3. Reason(R ) : The ...
Text Solution
|
- Consider the following molecules or ions : (i) CH(2) Cl(2) (ii) NH(4)^...
Text Solution
|
- निम्न प्रकार के संकरण निहित संकर यौगिकों की ज्यामितीय आकृति लिखिए - ...
Text Solution
|
- The d-orbitals involved in sp^(3)d^(2) or d^(2)sp^(3) hybridization of...
Text Solution
|
- The correct order of increasing s-character (in percentage) in the hyb...
Text Solution
|