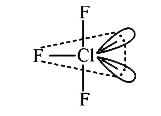
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In ClF(3), lone pair of electrons is present at equatorial position to...
Text Solution
|
- Statement : SF4 has lone pair of electron at equatorial position in pr...
Text Solution
|
- In BrF(3) molecule, the lone pair occupies equatorial position minimiz...
Text Solution
|
- Number of lone pair of electrons in XeF(4) is
Text Solution
|
- In BrF(3) molecule, the lone pair occupies equatorial position minimiz...
Text Solution
|
- In the structure of ClF(3), the number of lone pairs of electrons on c...
Text Solution
|
- How many lone pair of electrons are present on chlorine in ClF(3) mole...
Text Solution
|
- In the structure of ClF(3) , the number of lone pairs of electrons on ...
Text Solution
|
- Number of bonding electron pairs and number of lone pairs of electrons...
Text Solution
|