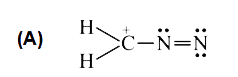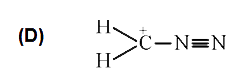A
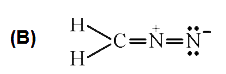
B
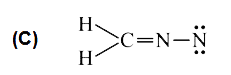
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the two do you think is more important contributor to the res...
Text Solution
|
- Benzene is resonance hybride of the following resonance contributors: ...
Text Solution
|
- In which of the following pairs of resonance contributors is the sturc...
Text Solution
|
- Which of the following is resonance contributors structores
Text Solution
|
- In the following sets of resonating structure, lable the major contrib...
Text Solution
|
- Do you think it is important to preserve languages?
Text Solution
|
- Which of the two do you think is more important contributor to the res...
Text Solution
|
- Why do you think forests are important?
Text Solution
|
- Which do you think is a more basic characteristic for classifying orga...
Text Solution
|