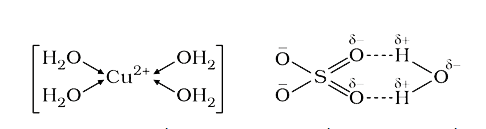
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- How many hydrogen-bonded water molecule(s) are associated in CuSO(4).5...
Text Solution
|
- How many hydrogen-bonded water molecule(s) are associated in CuSO(4).5...
Text Solution
|
- Cu SO4, 5H2 O में कितने जल - अणु हाइड्रोजन बंध द्वारा संगुणि...
Text Solution
|
- How many hydrogen-bonded water molecule (s) are associated in CuSO(4)....
Text Solution
|
- How many hydrogen-bonded water molecule (s) are associated in CuSO(4)....
Text Solution
|
- How many hydrogen bonded water molecule(s) are associated with CuSO(4)...
Text Solution
|
- CuSO(4) * 5H(2)O में कितने H(2)O अणु Cu से उप-सहसंयोजी बंध से जुड़े हो...
Text Solution
|
- Assertion : CuSO(4).5H(2)O has one hydrogen-bonded molecule of water. ...
Text Solution
|
- How many water molecules present in CuSO(4).5H(2)O are hydrogen bonded...
Text Solution
|