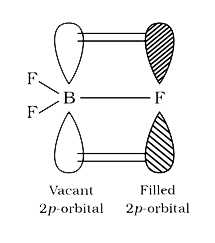
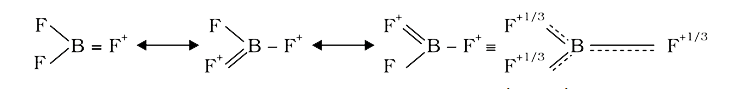A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The bond dissociation energy of B-F in BF(3) is 646 kJ mol^(-1) where...
Text Solution
|
- The bond dissociation energy of B-F in BF(3) is 646 kJ mol^(-1) where...
Text Solution
|
- The bond dissociation energy of B-F bond in BF3 is kJmol^(-1) whereas ...
Text Solution
|
- The bond dissociation energy of -F in BF(3) is 646 kJ mol^(-1) whereas...
Text Solution
|
- The bond dissociation energy of B-F in BF(3) is "646 kJ mol"^(-1) wher...
Text Solution
|
- The bond dissociation energy of B-F in BF(3) is "646 kJ mol"^(-1) wher...
Text Solution
|
- BF3 में B-F की आबन्ध वियोजन ऊर्जा 646kJ "mol"^(-1) है। जबकि CF4 में C-...
Text Solution
|
- The bond dissociation energy of B–F in BF3 is 646 kJ mol^(–1) whereas...
Text Solution
|
- The bond dissociation energy of B-F in BF(3) is "646 kJ mol"^(-1) wher...
Text Solution
|