A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
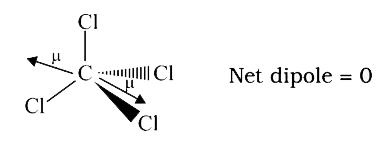
- Carbon tetrachloride has no net dipole moment because of
Text Solution
|
- Carbon tetrachloride has no net dipole moment because of
Text Solution
|
- H2O has net dipole moment while BeF2 has zero dipole moment because
Text Solution
|
- Carbon tetrachloride has no net dipole moment because of
Text Solution
|
- कार्बन ट्रेटाक्लोराइड का द्विध्रुव आघूर्ण शून्य होता है ,क्योकि
Text Solution
|
- H(2)O has a net dipole moment while BeF(2) has zero dipole moment beca...
Text Solution
|
- Carbon tetrachloride has no net dipole moment because of
Text Solution
|
- कार्बन टेट्राक्लोराइड का कुल द्विध्रुयव आघूर्ण शून्य होता है क्योकिं
Text Solution
|
- Carbon tetrachloride has no dipole moment because of
Text Solution
|