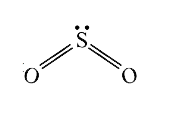
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which one of the following compounds has sp^(2) hybridization?
Text Solution
|
- Which one of the following compounds has sp^(2) hybridisation ? .
Text Solution
|
- Which has sp^2 -hybridization ?
Text Solution
|
- Which one of the following compounds has sp^(2) hybridization?
Text Solution
|
- Which of the following compounds contains one sp hybridized C atom?
Text Solution
|
- Which of the following has SP^(3) hybridization ?
Text Solution
|
- Which of the following compounds has (have) sp-hybridized carbon atom?
Text Solution
|
- Which one of the following compounds has sp^(2) hybridisation ?
Text Solution
|
- निम्नलिखित में से कौन से यौगिक में sp^(2) संकरण है ?
Text Solution
|