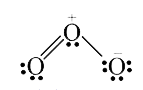
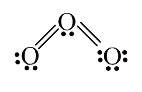
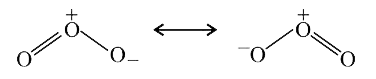
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Statement I: The electronic structure of O(3) is : Statement II...
Text Solution
|
- Asseration: The elctronic structure of O(3) is: Reason: structure is n...
Text Solution
|
- Statement I The electronic structure of O(3) is Statement II str...
Text Solution
|
- Statement I: The electronic structure of O(3) is : Statement II: struc...
Text Solution
|
- Statement I : Lysosomes are Membrane-bound structures. Statement II : ...
Text Solution
|
- वक्तव्य I O(3) की इलेक्ट्रॉनिक संरचना निम्न प्रकार है वक्त...
Text Solution
|
- Statement -I n - butane and iso butane are isomers Statement -II : b...
Text Solution
|
- Statement -I alkenes shows both structural and geometrical isomerism...
Text Solution
|
- Explain why the following two structures, I and II cannot be the major...
Text Solution
|