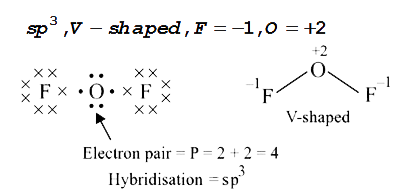Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Using the VSEPR theory, identify the type of hybridisation and draw th...
Text Solution
|
- Using the VSEPR theory, identify the type of hybisation and draw the s...
Text Solution
|
- Using the VSEPR theory, identify the type of hybridisation and draw th...
Text Solution
|
- Oxidation states of O in OF(2)" and "O(2)F(2) respectively are
Text Solution
|
- Using the VSEPR theory , identify the type of hydridisation and draw t...
Text Solution
|
- According to VSEPR theory, the shape of OF(2) molecule is .
Text Solution
|
- Deduce the structure of XeF(6) using VSEPR theory.
Text Solution
|
- VSPER सिद्धांत का प्रयोग करते हुए OF(2) अणु में संकरण के प्रकार की पहच...
Text Solution
|
- VSEPR Theory | Sp and Sp2 hybridisation
Text Solution
|