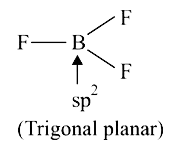
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The geometry and the type of hybrid orbitals present about the central...
Text Solution
|
- The geometry and the type of hybrid orbitals present about the central...
Text Solution
|
- The geometry and the hybirdisation present about the central atom in B...
Text Solution
|
- The geometry of a complex species can be understood from the knowledge...
Text Solution
|
- BF(3) में केन्द्रीय परमाणु की ज्यामिति व ऑर्बिटलों के संकरण का प्रकार ...
Text Solution
|
- The central atom of a molecule has 33% s-character in its hybrid orbit...
Text Solution
|
- BF(3) में केंद्रीय परमाणु के संगत ज्यामिति (geometry) तथा संकरित कक्षक...
Text Solution
|
- Predict the shapes of the following species and the type of hybrid orb...
Text Solution
|
- Predict the shapes of the following species and the type of hybrid orb...
Text Solution
|