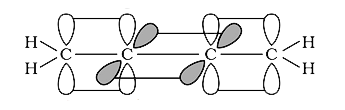
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Nodal planes of pi-bonds in CH(2)=C=C=CH(2) are located in,
Text Solution
|
- The nodal plane in the pi -bond of ethene is located in:
Text Solution
|
- The nodal plane is the pi -bond of ethene is located in :
Text Solution
|
- How many sigma bonds and pi bonds are present in CH(2)=C=CH(2) ?
Text Solution
|
- The nodal plane in the pi-bond of ethene is located in:
Text Solution
|
- How many sigma and pi bonds are present in (a) CH(3)-C-=N (b) CH(2)=C=...
Text Solution
|
- Indicate the sigma & pi bonds in (i) CH(2)Cl(2) (ii) CH(3)-C-=C-CH...
Text Solution
|
- The nodal plane in the pi-bond of ethene is located in:
Text Solution
|
- Nodal planes of pi-bonds in CH(2)=C=C=CH(2) are located in,
Text Solution
|